Major discovery or 'fake news'? Don't let cytometry assays fool you

With ‘fake news’ topping the headlines these days, we’re painfully aware that hearing just part of the whole story can lead to seriously wrong ideas that can have embarrassing or even disastrous consequences. The same is true when analysing cell populations.
Every individual cell has its own story to tell, so population averages and random samples are often misleading. When running assays on a cell imaging system, microplate reader or flow cytometer, can you be sure you are getting the whole truth? If not, it may be time to consider whole-well imaging.
Every cell counts in cytometry
To get a complete understanding of what’s happing in a cell culture, it makes sense to collect information from all the cells in the sample, without disrupting their behaviour and complex interactions. An average intensity measurement made with a microplate reader will not tell you the whole story; the same for microscope images that capture only a portion of the well, and flow cytometry of individual cells that have been stripped from the culture dish and separated from their neighbours.
Incomplete information can be perilously misleading and you may fail to notice serendipitous occurrences — like a rare interaction between cells that defies current theory, or an emerging clonal population that is expressing unusually high levels of a critical by-product. Goodbye serendipity, hello fake news!
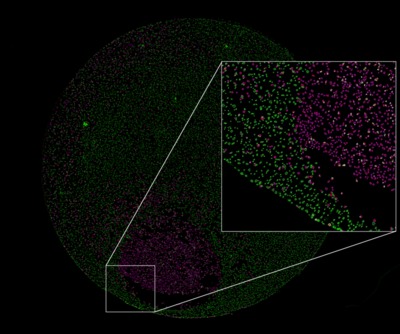
Do you know what you’re missing?
To get the most from assays performed on adherent cell cultures, you’ll want to know what is happening where — in every well of the plate, and in context with all the other cells in the culture. Otherwise, you are not getting the most out of the sample statistically and, more importantly, you are likely to miss valuable information about the following.
Cell position and distribution
Cells are dynamic entities; they move around, get vital cues from their neighbours, hang together in colonies and orient themselves towards or away from various stimuli. They rely on spatial information to know when to stop dividing and to function correctly in tissue maintenance, development and regeneration. Image-based information about the location and orientation of individual cells can therefore give you valuable clues about what is happening within the culture as a whole, and can be enabling for many adherent cell assays such as cell migration and wound healing. Because cell position and distribution can vary significantly with time and across the culture surface, the ability to assess the entire cell population at once may be essential to ensure reproducible and reliable results.
Cell confluence
Accurate assessment of cell confluence is often critical for pinpointing the optimal time window for actions such as initiation of imaging; addition of test compounds, reagents or fluorescent probes; and collection of end-point measurements. Uneven cell distribution or viability across the well can easily skew confluence assessments that are based on population sampling.
Sub-population responses
Cultured cell populations are typically heterogeneous with respect to many factors, including cell cycle phase, gene expression, viability, uptake of fluorescent probes and sensitivity to drugs or stimuli. As a result, population-averaged measures or sampling of only a subset of the total population may give misleading or ambiguous results. For example, does a low well-intensity reading collected with a plate reader reflect a high response from a small percentage of cells in the well, or a low-level response from the majority of cells? Are the cells imaged at the centre of the well behaving the same as those on the periphery? Without whole-well information, such questions are difficult if not impossible to answer with confidence.
Rare events and phenotypes
Capturing rare events and cell phenotypes is often essential for applications as diverse as clonal selection, elucidation of molecular mechanisms in disease and pathogen detection. It is easy to miss rare, transient or localised events if your detection system averages the responses or does not interrogate enough cells in a single pass. For the most statistically robust results, it makes sense to analyse every cell in the sample. This includes taking a look at cell morphology, which can tell you a lot about cell health, viability, function and developmental state.
Assay quality
It is possible that you waste a couple of months’ work thinking you discovered a powerful inhibitor, but the truth of the decrease in assay activity was an artefact due to the overly forceful addition of compound into the well. This is not noticed when you are imaging at the centre of the well while the damage is being initiated at the edge, where a liquid dispense head is positioned. This is just one example where looking at the entire well can provide important clues about assay quality, such as edge effects, mixing problems and more.
The bottom line is that every cell matters, because every cell is telling an important part of the story. If you capture just a few images from an entire well or rely on a population-averaged measurement, you are settling for a smaller effective sample size (read: less robust statistics) and literally throwing away valuable data!
Get the whole story with whole-well imaging
The answer? One of the best and least destructive tools for capturing all of the individual cell data from hundreds or even thousands of cells at a time is whole-well imaging. Reliable whole-well imaging, where the entire well area is captured in a single shot, has entered the mainstream only relatively recently. Even more recent is the emergence of whole-well imaging capability for multimode plate readers. Progress has been slow largely because of the technical challenges inherent in achieving sufficient resolution and definition at the well edges, without compromising too much on acquisition speed.
Increase the power of multimode microplate readers
Fortunately, the technical obstacles are being overcome, and there are now more types of systems on the market that offer whole-well imaging. Multimode microplate readers with whole-well imaging capability are particularly powerful because they give you the freedom to use a broad range of probes and detection modalities. Of course, not all cell imaging platforms perform the task as well or as efficiently as you need them to, particularly if your work involves kinetic analysis of living cells. It therefore pays to research what is available on the market and know which factors are critical to enabling high-quality whole-well imaging for both fixed and live-cell assays.
Discover more about the Spark Cyto live cell imaging microplate reader with real-time cytometry.
MRI scanner to advance medical breakthroughs at Monash
Siemens Healthineers' MAGNETOM Cima.X 3T is claimed to be Victoria's most advanced,...
Virtual pathology streamlines rapid onsite evaluation
Technology from Grundium, a specialist in digital imaging for pathology, has been shown to match...
Cannabis detected in breath from edibles
Researchers say they have made the first measurement of THC in breath from edible cannabis, in a...





