Catalyst enables efficient hydrogenation of nitriles
Achieving efficient and environmentally sustainable chemical processes is more important than ever before. Now, Japanese researchers have developed a nano-cobalt phosphide catalyst for the hydrogenation of nitriles that combines efficiency, cost-effectiveness, ease of handling and re-usability.
The hydrogenation of nitriles to primary amines is an important process that provides the building blocks for many everyday products and fuels. Primary amines are used as solvents and surfactants as well as in procedures for making dyes, pharmaceuticals and plastics.
Optimising nitrile hydrogenation in the interest of cost and environmental sustainability has led to numerous different types of catalyst being reported; however, when choosing a catalyst there is often a need to trade off different factors including performance and cost. Earth-abundant metal catalysts are cost-effective (due to their wide availability) but lack air stability, making them difficult to handle. In contrast, precious metal catalysts can be used under mild conditions but are prohibitively expensive for large-scale processes.
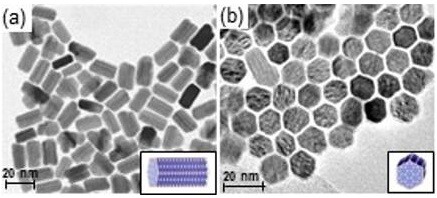
Researchers at Osaka University thus set out to develop a non-metal alloy heterogeneous cobalt phosphide catalyst that forms nanoparticles (nano-Co2P) that are stable in air and achieve efficient hydrogenation under mild conditions. Crucially, nano-Co2P can also be separated and re-used for subsequent reactions. Their work has been published in the journal Chemical Science.
“Despite being stable in air, our nano-cobalt phosphide catalyst has a very high activity,” said study author Min Sheng. “Its turnover number — which provides a measure of how productive a catalyst is — is 58,000. To put this in context, this is an improvement of up to 500-fold on previously reported catalysts for this type of reaction.”
Using the nano-Co2P catalyst, hydrogenation reactions could be carried out using hydrogen gas at ambient pressure, thus making nano-Co2P the first earth metal catalyst to be successfully used under mild conditions. This offers numerous advantages in terms of cost and safety. In addition, the catalyst was found to be effective for hydrogenating nitriles in a wide range of different organic molecules.
“Our study is the first example of a metal phosphide air-stable heterogeneous catalyst being used for this kind of reaction,” study lead author Takato Mitsudome concluded. “We believe that our findings will inspire a new direction in the catalysis of synthetic processes, supporting sustainable practices that protect the environment.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Likely source for Sydney's debris balls found
An investigation into the source of debris balls on Sydney and South Coast beaches has determined...
Polymers can act as a 'Trojan horse' for harmful chemicals
The scientific community has long believed that polymers are too big to migrate out of products...
Forever chemicals can be safely incinerated
A team of international scientists has shown how per- and polyfluoroalkyl substances (PFAS) can...




