Scientists reveal the structure of benzene
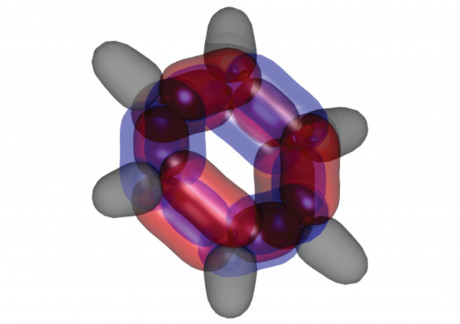
Australian scientists have solved what they claim is one of the fundamental mysteries of chemistry — and the result may have implications for future designs of solar cells, organic light-emitting diodes and other next-gen technologies.
Ever since the 1930s, debate has raged inside chemistry circles concerning the fundamental structure of the organic chemical compound benzene. It is a debate that in recent years has taken on added urgency, because benzene — which comprises six carbon atoms matched with six hydrogen atoms — is the smallest molecule that can be used in the production of opto-electronic materials, which are revolutionising renewable energy and telecommunications technologies. It is also a component of DNA, proteins, wood and petroleum.
The controversy around the structure of the molecule arises because, although it has few atomic components, it exists in a state comprising not just four dimensions — like our everyday ‘big’ world — but 126. Measuring a system that complex — and tiny — has until now proven impossible, meaning that the precise behaviour of benzene electrons could not be discovered. Without that information, the stability of the molecule in tech applications could never be wholly understood.
Now, scientists led by Timothy Schmidt from the ARC Centre of Excellence in Exciton Science and UNSW Sydney have succeeded in unravelling the mystery — and the results, published in the journal Nature Communications, came as a surprise.
Working with colleagues from UNSW and CSIRO’s Data61, Prof Schmidt applied a complex algorithm-based method called dynamic Voronoi Metropolis sampling (DVMS) to benzene molecules in order to map their wave functions across all 126 dimensions. Using a mathematical algorithm developed by Data61’s Dr Phil Kilby, the scientists were able to partition the dimensional space into equivalent ‘tiles’, each corresponding to a permutation of electron positions.
The scientists were particularly keen to understand the ‘spin’ of the electrons. All electrons have spin — it is the property that produces magnetism, among other fundamental forces — but how they interact with each other is at the base of a wide range of technologies, from light-emitting diodes to quantum computing.
“What we found was very surprising,” Prof Schmidt said. “The electrons with what’s known as up-spin double bonded, while those with down-spin remained single.
“That isn’t how chemists think about benzene. Essentially it reduces the energy of the molecule, making it more stable, by getting electrons, which repel each other, out of each other’s way.
“That wasn’t what we were expecting, but it could be good news for future technological applications.”
Co-author Kilby added, “Although developed for this chemistry context, the algorithm we developed, for ‘matching with constraints’, can also be applied to a wide variety of areas, from staff rostering to kidney exchange programs.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Polymers can act as a 'Trojan horse' for harmful chemicals
The scientific community has long believed that polymers are too big to migrate out of products...
Forever chemicals can be safely incinerated
A team of international scientists has shown how per- and polyfluoroalkyl substances (PFAS) can...
'Nuclear spin microscopy' based on quantum sensors
Researchers have used quantum sensors to extend magnetic resonance imaging to the realm of...




