Selective catalyst for propane dehydrogenation developed
Japanese scientists have developed an ultrastable, selective catalyst to dehydrogenate propane — an essential process to produce the key petrochemical substance of propylene — without deactivation, even at temperatures of more than 600°C. Their work has been published in the journal Nature Communications.
Propylene is an important raw material for plastics, synthetic rubber, surfactants, dyes and pharmaceuticals. In recent years, there has been an increased demand for propylene produced from cheaper, shale-originated propane. Reaction temperatures of more than 600°C are necessary to obtain sufficient propylene yields, but under these harsh conditions, severe catalyst deactivation is inevitable due to carbon deposition and/or sintering. Catalysts in practical use must therefore be regenerated either continuously or in short cycles, making the process inefficient and costly.
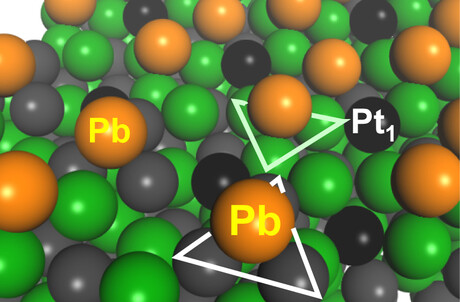
The Japanese research team focused on the intermetallics (PtGa) of platinum (Pt) and gallium (Ga), which have unique properties and structures. PtGa has a high thermal stability and its structure does not change even under high temperatures. It is also known to have two kinds of catalytic sites on its surface: one with three Pt atoms (Pt3 site) and one with single-atom-like isolated Pt (Pt1 site).
The group hypothesised that if the Pt3 sites — which facilitate carbon deposition in addition to producing propylene — are disabled to allow only the Pt1 sites to function, the catalyst will be ultrastable and also able to prevent carbon deposition. The group tried various metals and catalyst synthesis methods to make only the Pt1 sites function.
The newly developed catalyst (PtGa-Pb/SiO2), which is silica-supported and made by adding lead (Pb) to the surface of PtGa, exhibits no deactivation when dehydrogenating propane at 600°C. The catalyst maintained the initial conversion rate of 30% for 96 hours after the reaction started, which is significantly more stable than conventional catalysts. Propylene selectivity is as high as 99.6% with few side reactions, including carbon deposition.
The results claim that the catalyst produced the world’s best performance at temperatures of 580°C or higher, with a life span that is more than twice as long as the previous reported record longevity for such catalysts. Furthermore, the catalyst can be produced as cheaply as conventional catalysts. Structural analysis confirmed Pt3 sites, not Pt1 sites, were covered and disabled by Pb, as the researchers expected.
“Our finding could lead to a more efficient and cheaper industrial process to produce propylene from propane without the need for catalyst regeneration — which is far superior in selectivity and stability than conventional ones,” said Hokkaido University Associate Professor Shinya Furukawa, a member of the research team. “Moreover, this method could be applicable to dehydrogenation of other lower alkanes such as ethane and isobutane, thus contributing to the petrochemical industry’s development.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Polymers can act as a 'Trojan horse' for harmful chemicals
The scientific community has long believed that polymers are too big to migrate out of products...
Forever chemicals can be safely incinerated
A team of international scientists has shown how per- and polyfluoroalkyl substances (PFAS) can...
'Nuclear spin microscopy' based on quantum sensors
Researchers have used quantum sensors to extend magnetic resonance imaging to the realm of...




