Switching oxygen molecules on and off
It is now possible to selectively switch individual oxygen molecules ‘off’ and ‘on’, from a non-reactive to a reactive state, using a special force microscope. The process was discovered by researchers at Austria’s TU Wien and has been published in the journal Proceedings of the National Academy of Sciences.
As noted by Martin Setvin, a member of the research team, there are several ways to switch a stable, non-reactive O2 molecule into a reactive state. “You can increase the temperature — that happens when you burn things,” he said. “Alternatively, you can add an additional electron to the molecules — this also makes them chemically active.”
The researchers created their own activation method when they studied the oxygen molecules on the surface of a titanium oxide crystal at extremely low temperatures. Among its many interesting properties, titanium oxide is a photocatalyst, which means that it can induce chemical reactions when irradiated with light.
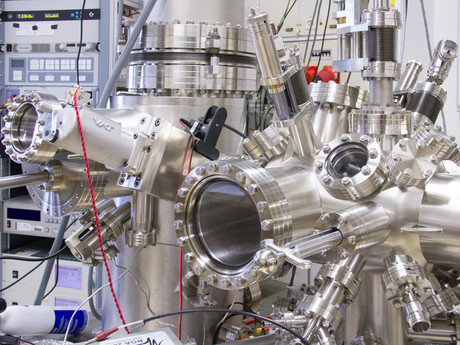
This change in reactive state was observed using an atomic force microscope, purchased by team leader Professor Ulrike Diebold, who explained how the device works.
“A tiny needle is vibrated and moved across the surface. When the atoms at the very end of the tip come close to the surface, the tip feels a force and the oscillation changes. From this tiny change, one can create an image showing where the atoms are,” said Professor Diebold.
“Essentially, the reactive oxygen molecules that have an extra electron exert a stronger force on the tip than the unreactive ones, and thus we can distinguish them.”
The researchers found that is possible to inject an additional electron to an individual oxygen molecule with the same tip, and then observe the transition from the inactive to the active state. The same process occurs when the surface of the titanium oxide is irradiated with light — electrons are liberated inside the material and can come to the surface to activate one of the oxygen molecules.
“Whether we add an electron using the microscope or by irradiating the titanium oxide, the end result is the same,” said Professor Diebold. “Our method gives us a whole new level of control over this process and opens up new possibilities for investigating the inner workings of photocatalysts.”
'Phantom chemical' in drinking water finally identified
Researchers have discovered a previously unknown compound in chloraminated drinking water —...
Flinders facility to use the micro realm to understand the past
AusMAP aims to revolutionise the ways scientists address key questions and grand challenges in...
A new, simpler method for detecting PFAS in water
Researchers demonstrated that their small, inexpensive device is feasible for identifying various...




