What happens when a chemical bond is broken?
That question was recently answered with the help of a so-called free electron X-ray laser, which makes it possible to follow in real time how bindings in a molecule are changed and broken. The study, published in Science, found, among other things, evidence of a much-discussed intermediate state before molecules bind to or leave a metal surface. The possibility of monitoring at the molecular level how the electron structure changes in a chemical reaction creates entirely new opportunities for investigating and understanding key chemical processes in detail.
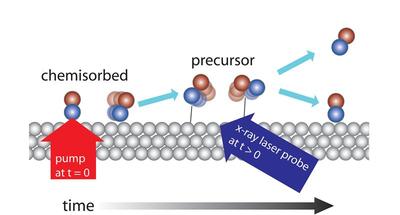
“To identify and characterise short-lived intermediate states in chemical reactions on the surface of metals has long been a dream,” says Henrik Öström at the Department of Physics, Stockholm University, who is part of the international research team that carried out the study. “With the new free electron X-ray laser at SLAC, we have shown that dreams can become reality and managed to identify a short-lived intermediate state when the bindings of CO molecules to a metal surface are broken or created.”
SLAC (Stanford Linear Accelerator Center) was long a flagship of particle physics, where electrons were accelerated to nearly the speed of light in a three-kilometre-long linear accelerator. Now the accelerator has been rebuilt to generate, instead, powerful ultra-short (10-100 femtosecond) pulses of X-ray beams with a wavelength that makes it possible to examine the surroundings of a molecule down to the level of an individual atom. The pulses are sufficiently short to provide a snapshot of the electron distribution around the atom. By varying the delay between the start of a reaction and when the distribution of electrons is monitored with the X-ray pulse, these scientists can create a suspended-time image of changes in the course of the reaction.
“A first challenge was whether the incredibly powerful pulse would destroy the sample,” explains Anders Nilsson, a professor of synchrotron-light physics at SLAC and an adjunct professor at Stockholm University. “However, it turned out to be entirely possible to adjust the experiment in a way that enabled us to make our measurements.”
In the experiment, CO molecules were dosed onto a metal surface of ruthenium, which is used in automobile catalytic converters, for instance. CO binds strongly to the surface but can be made to let go by heating up the surface, which was done with a pulse from an optical laser. By starting the reaction for all the molecules at the same time, the team got a sufficient number of molecules to simultaneously enter a state where they have almost let go of the surface but still have a weak binding to it. From this short-lived state, the molecules can then continue out into a gas phase or renew their bond when the surface cools down again.
“Scientists have long speculated whether such a state, a so-called ‘precursor’, exists. The new experiment is the first to directly show its existence,” says Lars G M Pettersson at the Department of Physic, Stockholm University. These studies will now go on to more complex reactions of interest to the field of synthetic fuels, among other applications.
Besides the researchers from Stockholm University were scientists from Stanford University, SLAC, the University of Hamburg, the Technical University of Denmark, the Helmholtz Center in Berlin, and the Fritz Haber Institute in Berlin.
New scanning method observes lung function in real time
Using the new scanning method, the team are able to reveal the parts of the lung that air...
'Phantom chemical' in drinking water finally identified
Researchers have discovered a previously unknown compound in chloraminated drinking water —...
Flinders facility to use the micro realm to understand the past
AusMAP aims to revolutionise the ways scientists address key questions and grand challenges in...




