2D electrolytes: a new type of intelligent material
Intelligent materials can adapt their properties depending on changes in their surroundings and can be used in everything from self-healing mobile phone screens to shape-shifting aeroplane wings and targeted drug delivery.
Now, researchers from the National University of Singapore (NUS) have created an intelligent material that has the structure of a two-dimensional (2D) material but behaves like an electrolyte — and it could be a new way to deliver drugs within the body.
Just like traditional electrolytes, these ‘2D electrolytes’ dissociate their atoms in different solvents, and become electrically charged. Furthermore, the arrangement of these materials can be controlled by external factors, such as pH and temperature, which is ideal for targeted drug delivery. The 2D electrolytes also show promise for other applications that require a material to be responsive to environmental changes, such as artificial muscles and energy storage.
The team behind the 2D electrolytes comprised NUS researchers from the university’s Centre for Advanced 2D Materials (CA2DM), Department of Physics and Department of Materials Science and Engineering. Their results have been published in the journal Advanced Materials.
Changing the behaviour of 2D materials
In materials science, a 2D material is a solid material that exists in a single layer of atoms. It can be thought of as an atomically thin sheet that has a specific height and width, but effectively no depth; hence, it is essentially two-dimensional. On the other hand, an electrolyte is a substance that produces an electrically conducting suspension when dissolved in a solvent, such as water.
There are numerous 2D materials in existence today, and electrolytic behaviour has been well established in countless other compounds. However, the results from the NUS researchers show the first instance of materials that have both 2D structure and properties of electrolytes, with a particular trend to shapeshift their form reversibly in liquid medium. The team achieved this feat by using organic molecules as reactive species to add different functionalities to 2D materials such as graphene and molybdenum disulfide (MoS2).
“By adding different chemical groups that become positively or negatively electrically charged in solvents, we altered traditional 2D materials and came up with a novel class of smart materials that have their electronic properties controlled by morphological conformation,” explained Professor Antonio Castro Neto, Director of CA2DM and leader of the study.
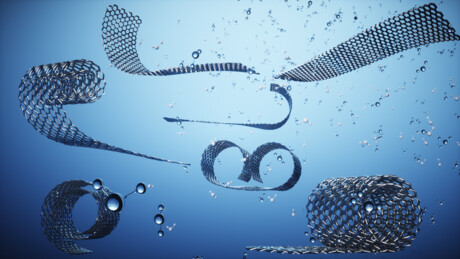
From a flat sheet to a rolled-up scroll
A major breakthrough of the research was that the orientation of the 2D electrolytes could reversibly change by tweaking the external conditions. Currently, the electrical repulsion between the surface charge in a 2D material leads to it being laid out in a flat sheet. By altering the pH, the temperature or the ionic concentration of the suspensions, the researchers demonstrated the ability of the 2D electrolyte sheet to shapeshift and form scroll-like arrangements. These experimental results are supported by detailed theoretical analysis in which they explain the physical mechanism behind the scroll formation and stability.
These scroll orientations have such a small diameter that they could be described as one-dimensional (1D), leading to different physical and chemical properties. Moreover, this transition from 2D to 1D is reversible by altering the external conditions back to their original values.
“One can think of 2D electrolytes as the higher dimensional analogues of 1D electrolytes, commonly known as polyelectrolytes,” said Prof Castro Neto. Important examples of polyelectrolytes include many biologically relevant materials, such as DNA and RNA.
“When acids, bases or salts are added, these electrically charged polymers also undergo conformational transitions from molecular chains that are 1D to globular objects of 0D, and vice versa. Our 2D electrolytes, in analogy with polyelectrolytes, show reversible transitions from 2D to 1D, as a function of external factors. As stimuli-responsive materials, they are suitable for the creation of cutting-edge technology.”
Next steps
Discovering this class of materials has opened up new areas of exploration for materials scientists. As noted by Prof Castro Neto, “There is an uncountable number of ways to functionalise graphene and other 2D materials to transform them into 2D electrolytes. We hope that our work will inspire scientists from different fields to further explore the properties and possible applications of 2D electrolytes.
“We anticipate that as 2D electrolytes have similarities with biological or natural systems, they are capable of spontaneously self-assembling and cross-linking to form nanofibres that are promising for applications in filtration membranes, drug delivery and smart e-textiles.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Chewing gum can shed microplastics into saliva, study finds
Most of the microplastics detached from gum within the first two minutes of chewing, with the act...
Widespread applications of shake flask off-gas analysis across biotechnology
Despite the emergence of more sophisticated bioreactor systems, shake flasks continue to play a...
Scientists may finally know what makes Mars red
The water-rich iron mineral ferrihydrite may be the main culprit behind Mars's reddish dust,...




