A reversible adhesive that's easy to unstick
Some adhesives may soon have a metallic sheen and be particularly easy to unstick. Researchers from Germany’s Max Planck Institute for Intelligent Systems are suggesting the use of gallium as one such adhesive.
By inducing slight changes in temperature, the scientists can control whether a layer of gallium sticks or not. A reversible adhesive of this kind could have applications everywhere that temporary adhesion is required, such as industrial pick-and-place processes, transfer printing, temporary wafer bonding or moving sensitive biological samples.
The principle is simple: above 30°C, gallium metal is liquid; below 30°C, it is solid. If a drop of liquid gallium is introduced between two objects and then cooled to less than 30°C, the gallium layer solidifies and sticks the two objects together. When it is time to separate the objects, the temperature is raised to transition the gallium layer to its liquid state and they can be pulled apart with a small amount of unloading force. Gallium actually works in a similar fashion to hot glue, the difference being that far less heating and cooling is required, it lifts more easily and cleanly from the surface, it is highly repeatable and it is electrically conductive.
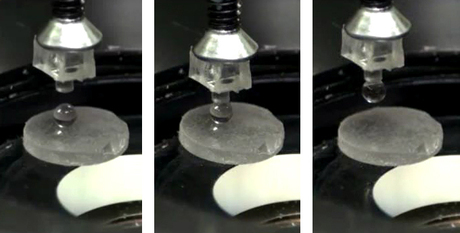
Scientists working with Metin Sitti, director at the Max Planck Institute, conducted their experiments by wetting the tip of a cylindrical elastomer rod with liquid gallium. They then brought the gallium droplet into contact with different materials such as glass, plastic and gold. After cooling the tip to 23°C, they found that the solidified gallium formed a strong bond between the elastomer and each of the materials. They also found that the greater the difference of the binding power between the liquid and solid state, the easier it was to reverse and switch the adhesive effect.
The team deliberately tested gallium on particularly rough and damp surfaces — surface conditions which have “showed up as major weaknesses of reversible micro/nanostructured adhesives proposed recently”, according to Sitti. This is because adhesives that have yielded strong binding values on rough or wet surfaces to date have always had poor reversibility.
Gallium, however, was found to be effective in damp conditions, even underwater. Its binding power and reversibility when wet were reduced compared to dry conditions, but they still remained relatively strong for a wide range of applications. This makes it suitable for biological applications, noted Sitti, perhaps one day moving individual cells, tissue samples or even organs in laboratory or hospital settings.
Another possible field of application is industrial manufacturing, especially where fragile components such as ultrathin graphene membranes or tiny electronic chips are involved. These components could be picked up by gallium-coated grippers and then set down at the precise location where they are required, eg, a circuit board. This kind of assembly technology is called ‘pick and place’ and is typically based on the use of vacuum suction.
“Wetting an object with a metallic liquid such as gallium that forms a bond when cooled slightly is a far more gentle process for fragile materials than sucking them up using a vacuum,” claimed Sitti, adding that the new methods are also more energy efficient. Once an object adheres to the gallium layer, no more energy is required to sustain the adhesive bond. Only when it is time to reverse the adhesion must the metal be quickly heated to 30°C. The vacuum technique, however, requires the constant use of suction in order to maintain the adhesive effect.
Sitti sees robotics as another possible application for this adhesive. For example, climbing robots such as those that may one day ascend wind turbines for maintenance purposes could benefit from reversible adhesives. By activating the adhesive, the robot foot would be fixed to the wall of the turbine and, for the next step, the adhesive layer between the foot and the wall would be briefly heated by means of an integrated heating element.
To achieve rapid heating and cooling in their tests, the team connected a Peltier element to their experiment set-up. This element can either release or absorb heat when an electric current is applied. For practical applications in the future, the scientists anticipate that the adhesive bond could also be reversed using infrared radiation remotely or using electrical Joule heating by integrating conductive wiring to the surface.
Another consideration for practical applications is that the material should be able to be used for as many cycles as possible without the need to replace it. Gallium conforms to this requirement as the liquid metal lifts completely from the substrate with proper loading and unloading conditions. No residues are left on the surface and the adhesive loses none of its own substance.
“Good adhesives are generally hard to separate from the substrate,” stated Sitti, explaining that in gallium’s case, the material forms a fine oxide layer in air. This shell of gallium oxide retains the gallium and ensures that no residues are left behind when the adhesion is reversed.
Sitti’s team has started exploring some of these potential applications already, while also working to optimise the technique. The scientists also plan to study alloys of gallium with other metals such as indium, but they will be watching closely to ensure that the melting point is close to normal ambient temperature.
Chewing gum can shed microplastics into saliva, study finds
Most of the microplastics detached from gum within the first two minutes of chewing, with the act...
Widespread applications of shake flask off-gas analysis across biotechnology
Despite the emergence of more sophisticated bioreactor systems, shake flasks continue to play a...
Scientists may finally know what makes Mars red
The water-rich iron mineral ferrihydrite may be the main culprit behind Mars's reddish dust,...




