No scale, no problem: weighing tiny samples in fluid environments
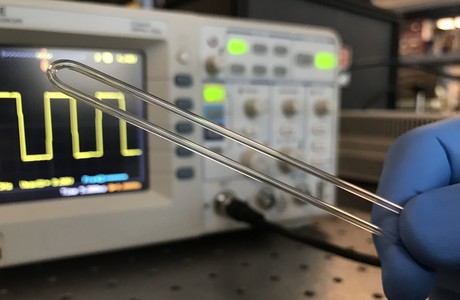
Scientists at the University of California, Riverside have turned a simple glass tube into a sensor to measure the mass, volume and density of microgram-sized biological samples in fluid. Published in the journal PLOS ONE, this inexpensive device could be used to speed up chemical toxicity tests, shed light on plant growth, develop new biomaterials and more.
While weight is one of the most fundamental and important measures of an object, weighing tiny biological samples in their native liquid environments is not possible with conventional scales. Doctoral student Shirin Mesbah Oskui sought to address this, developing a sensor out of a small piece of glass tubing bent into a ‘U’ shape and attached to an inexpensive speaker. The speaker vibrates the glass at its resonance frequency, which is a function of the overall mass of the tube. When a sample is pumped into the tube, the resonance frequency changes, allowing the sample’s mass, density and volume to be calculated.
As proof of concept, Mesbah Oskui and her fellow scientists turned to an important research area: toxicology. Many chemicals in use today are yet to be fully evaluated for their risk to human health and the environment, primarily because toxicology tests on traditional animal models are expensive, time-consuming and labour-intensive. And while the introduction of tests using tiny zebrafish embryos is speeding things up, scientists have not yet been able to weigh these embryos in their native fluid environment.
The development of the glass tube sensor has changed this, enabling the researchers to measure mass changes in zebrafish embryos as they reacted to toxic silver nanoparticles. The team additionally used the sensor to measure density changes in seeds undergoing rehydration and germination, as well as degradation rates of biomaterials used in medical implants.
“Biodegradable materials can be used to cover a wound after surgery, but if they degrade too quickly they can leave an open wound and if they don’t degrade quickly enough they can cause complications,” said graduate student Heran Bhakta. “Previously, tracking the degradation of biomaterials in a fluid environment has been a painstaking and error-prone process in which the biomaterial sample must be removed, cleaned, weighed and replaced on an ongoing basis. In contrast, our sensors enable us to measure the degradation of even the tiniest biomaterials samples continuously as they break down in a biological fluid.”
Assistant Professor William Grover, who led the research in collaboration with Mesbah Oskui, said the automation, portability and low cost of the technique make the sensors well suited to applications in the field or in resource-limited settings.
“Our technique is so versatile, because all objects have fundamental properties like weight,” he said. “I am excited to see how the sensors will be used by other researchers to address scientific problems in many other fields.”
Chewing gum can shed microplastics into saliva, study finds
Most of the microplastics detached from gum within the first two minutes of chewing, with the act...
Widespread applications of shake flask off-gas analysis across biotechnology
Despite the emergence of more sophisticated bioreactor systems, shake flasks continue to play a...
Scientists may finally know what makes Mars red
The water-rich iron mineral ferrihydrite may be the main culprit behind Mars's reddish dust,...




