PCR alternative offers diagnostic testing in a handheld device
Researchers at the University of Connecticut have developed a diagnostic platform that uses similar techniques to polymerase chain reaction (PCR), but within a handheld device rather than several benchtop machines. With advantages of cost, speed and portability over traditional methods, the new technology could make powerful diagnostics for infectious disease more widespread.
Some of the most common tests for infectious disease detect either viral material called antigens or proteins produced by the immune system in response to infection known as antibodies. Despite the reliability, speed and widespread availability of these tests, which include the now prevalent COVID-19 rapid antigen tests, laboratory-based PCR still holds an advantage in terms of accuracy, which can be nearly 100%.
PCR’s strength as a diagnostic tool comes from its ability to detect the genetic material, such as RNA, of pathogens directly. This approach provides greater specificity, meaning it is less likely to mistake non-pathogenic particles for the target and produce a false positive result. PCR also amplifies the target it is looking for to make it sensitive to smaller amounts of pathogenic material, meaning even a low level of infection can be detected.
But PCR requires highly trained staff and costly equipment, hindering its availability — especially in low-resource settings. This means patients and healthcare providers often need to choose between technology that is easy to access and technology with high performance. Professor Changchun Liu and his colleagues at the University of Connecticut aimed to break down this dichotomy.
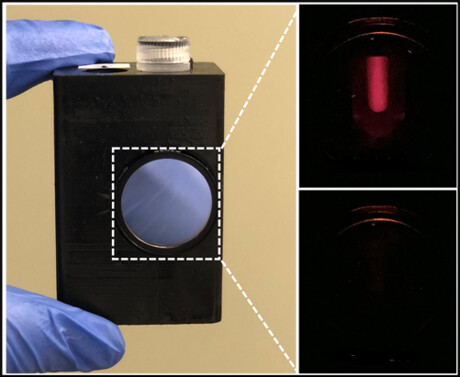
The team’s proposed solution is the lab-in-a-magnetofluidic tube (LIAMT) — a device composed of a 1.5 mL tube containing a 3D-printed insert and a wireless handheld processor. Whereas PCR involves the use of several benchtop machines, LIAMT is a portable one-stop shop that performs similar processes using drastically slimmed down hardware and simplified user training. One major difference between the two approaches is how LIAMT isolates genetic material from biological samples.
Whereas traditional techniques rely on fast-spinning machines called centrifuges to separate lighter nucleic acids from the rest of the sample, LIAMT relies on magnetism. Tiny magnetic beads within the tube stick to viral RNA in the sample through the attraction of electric charges on the molecules. Then, after a user places the tube within the handheld processor, a magnet inside the device pulls the RNA-bound beads through several washing steps in the tube, filtering out unwanted material.
Once genetic material is isolated, PCR copies specific stretches from a virus’s genetic code millions to billions of times over so it can be detected more easily. This step entails many cycles of heating and cooling to facilitate the separation of paired nucleic acid strands and their subsequent duplication, which requires specialised thermal cycling equipment.
LIAMT instead incorporates a recent PCR alternative that employs special proteins that are able to split up and amplify nucleic acid strands at a low and constant temperature. After amplification, the wireless LIAMT device heats the sample by a few degrees, melting a wax barrier in the tube and releasing a solution of CRISPR enzymes that results in the emission of a fluorescent signal after they bind to their target. If enough viral RNA was present in the initial sample, then that signal can be viewed through a small window in the LIAMT device, indicating a positive test result.
Free of the burden of a centrifuge and thermal cycler, the portable LIAMT could be used in a clinic or in a home. The diagnostic tool can also produce results in about an hour, which is much faster than PCR usually takes, as test sites often do not have the necessary equipment (meaning samples need to be shipped offsite for analysis).
To test how their tool fared against PCR, the researchers obtained 32 swab samples from patients tested for SARS-CoV-2 and 41 blood plasma samples from patients tested for HIV. They used both LIAMT and traditional PCR to search for viral RNA within the samples and compared the outcomes.
As revealed in the journal Advanced Science, the researchers found the sensitivity and specificity of LIAMT to be promising for both viruses. When compared to PCR, LIAMT’s designations of whether samples were positive or negative aligned for 29 of the 32 swab samples and 40 of the 41 plasma samples.
Encouraged by these results, Liu seeks to continue developing the LIAMT platform, fine-tuning its performance and usability. He is particularly motivated by the possibility of improving care for people with HIV, who often need to undergo routine testing to receive treatment, which involves hospital visits, having blood drawn and days of waiting for results.
“With our device, we used the volume you could get from a finger prick to produce accurate HIV test results quickly,” Liu said. “Patients could take these tests at a local point-of-care centre or at home and receive the medication they need more quickly.”
Droplet microfluidics for single-cell analysis
Discover how droplet microfluidics is revolutionising single-cell analysis and selection in...
Urine test enables non-invasive bladder cancer detection
Researchers have developed a streamlined and simplified DNA-based urine test to improve early...
Platform helps accelerate synthetic and metabolic workflows
Ultrahigh-throughput, droplet-based screening had long been on Biosyntia's radar for its...




