Controlling protein synthesis with light
The production of proteins at distinct times and locations regulates cell functions, such as cell development and differentiation into specific cell types. But while the usual mode for protein synthesis uses transfer RNA (tRNA), messenger RNA (mRNA) and ribosomes to assemble the amino acid chains in the right order, the ability to exert spatiotemporal control over protein synthesis would be useful.
Now, Takashi Ohtsuki and colleagues at Japan’s Okayama University have shown that they can prevent protein synthesis reactions from taking place using a photoresponsive molecular cage. Brief exposure to light releases key protein synthesis molecules from the cage without damaging them, so that protein synthesis takes place at the time and location of irradiation. The results have been published in the journal Nature Communications.
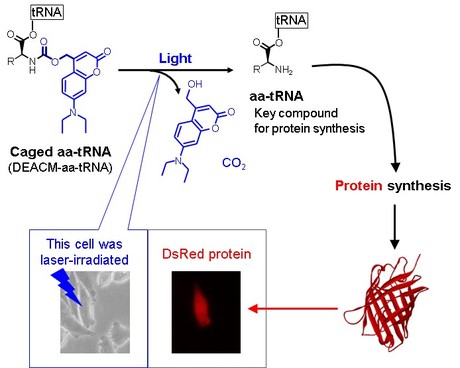
The scientists used an aminoacyl-transfer RNA (aa-tRNA), a molecule that helps decode messenger RNA so that protein synthesis can occur. Previous work had successfully inhibited protein synthesis by protecting aa-tRNA with a molecular cage of nitroveratryloxycarbonyl group. However, the 30 minutes of light exposure required to release the aa-tRNA from the cage caused damage that then inhibited the protein’s function.
Instead, the team used (7-diethylaminocoumarin-4-yl) methoxycarbonyl (DEACM) group. The DEACM group protects the aa-tRNA so that it cannot interact with the molecules necessary for protein synthesis. On exposure to light, the DEACM-aa-tRNA degrades into separate components releasing the aa-tRNA. While the caged aa-tRNA was stable for at least 4 hours, irradiating for just 20 seconds released the aa-tRNA.
The researchers demonstrated the light-responsive aa-tRNA release by irradiating a gel containing green fluorescent protein mRNA and the complex through a mask in the shape of a smiley face. No fluorescence was observed until the gel was irradiated with 405 nm (blue) light, soon after which fluorescence was observed simulating the shape of the mask.
As well as showing light responsive protein synthesis in vitro, gels and liposomes, the scientists also inserted the caged aa-tRNA in Chinese hamster ovary cells. Following 5 hours of incubation, the researchers observed fluorescence of photo-dependently synthesised DsRed protein in the irradiated cells.
“This method of spatiotemporally photocontrolling translation offers a promising approach for investigating the relationship between local translation and biological functions,” the researchers wrote. As well as a tool for protein synthesis investigations, the team suggest it could also be used to insert artificial amino acids into proteins in a controlled manner for further studies.
Blood test could be used to diagnose Parkinson's earlier
Researchers have developed a new method that requires only a blood draw, offering a non-invasive...
Cord blood test could predict a baby's risk of type 2 diabetes
By analysing the DNA in cord blood from babies born to mothers with gestational diabetes,...
DNA analysis device built with a basic 3D printer
The Do-It-Yourself Nucleic Acid Fluorometer, or DIYNAFLUOR, is a portable device that measures...





