Clinuvel success in Scenesse trial
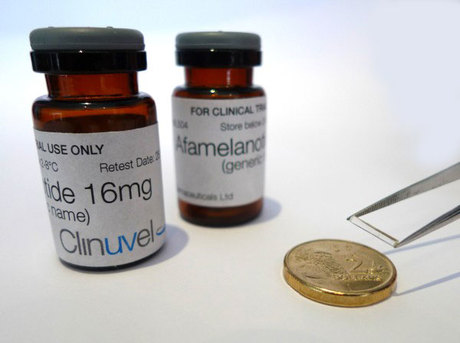
Clinuvel Pharmaceuticals (ASX:CUV) has published results of a phase IIa study of its Scenesse implant that show it to be a more effective treatment for the skin disorder vitiligo than existing therapies.
Vitiligo is a skin disorder where the melanocytes – the pigment producing cells in the skin – become dysfunctional and leave patches with little or no pigmentation.
The condition affects around 45 million people worldwide, and the standard existing treatment involves exposing the skin to narrowband ultraviolet B (NB-UVB) light to slow depigmentation and stimulate repigmentation – i.e. a tan to bring the depigmented regions to a similar colour with the rest of the skin.

The existing treatment is given in two or three sessions per week over the course of 12 to 18 months, but the response rate is low and repigmentation often incomplete.
The phase IIa trial, CUV102, involved NB-UVB in combination with Scenesse, which is an implant with 16mg of afamelanotide, which stimulates the production of melanin in the skin.
It was conducted at three locations in the United States and involved 54 patients, 41 of whom completed the trial. Five had to withdraw because the repigmentation was too intense.
The primary end point of the trial was reached, and it found that the combination treatment achieved faster and greater repigmentation than NB-UVB alone.
Safety was also acceptable, with no significant side effects or safety issues reported.
“I think this is an exciting, major advancement for vitiligo, a disease for which we do not have adequate effective treatments,” said Dr Mark Lebwohl, Professor and Chair of Dermatology at Mount Sinai Hospital in New York and an investigator on the CUV102 study.
“We were thrilled with the speed at which the pigment returned. Until now all treatments had taken many years to achieve repigmentation in vitiligo and patients had great difficulty complying with the rigours of light treatment regimes. We finally have a treatment that allows us to repigment patients much more quickly.”
Clinuvel’s acting Chief Scientific Officer, Dr Dennis Wright said the combination therapy could simplify treatment of vitiligo.
“This has the potential to substantially reduce the current regimen of 18 months of NB-UVB treatment thrice per week, while the new treatment achieves a better outcome for patients, enabling them a life without being stigmatised.
The treatment was found to be most effective in individuals with olive skin, or skin phenotype IV. “Encouragingly, patients with skin phototype IV and above respond very well to treatment. These are patients for whom vitiligo can be the most visible and have the most devastating impact on quality of life,” said Wright.
“Overall, the feedback from patients and physicians has been extremely positive as to the potential of Scenesse becoming the standard of care in the treatment of vitiligo.” Following the trial, Clinuvel is considering advancing to a phase IIb study of vitiligo in Europe and Asia.
Currently Scenesse is also undergoing phase III trials as a treatment for erythropoietic protoporphyria, and the European Medicines Agency is currently reviewing the company’s application for marketing authorisation.
Clinvuel’s (ASX:CUV) share price jumped up 6.75% in today’s trading, up 12c to $1.98.
Breakthrough drug prevents long COVID symptoms in mice
Mice treated with the antiviral compound were protected from long-term brain and lung dysfunction...
Antibiotics hinder vaccine response in infants
Infants who received antibiotics in the first few weeks of life had significantly lower levels of...
Colossal announces 'de-extinction' of the dire wolf
Colossal Biosciences has announced what it describes as the rebirth of the dire wolf, which would...




