Needle-free vaccine to target strep A infections
Researchers from Griffith University’s Institute for Glycomics will soon begin Phase 1 clinical trials investigating a needle-free vaccine targeted at Streptococcus A infection.
The trials have been made possible thanks to a licensing deal with China’s Olymvax Biopharmaceuticals, which will see the biopharma company gain commercialisation rights in Greater China should the outcome be successful.
Strep A bacteria are responsible for a wide range of illnesses — from common infections like strep throat and skin sores to toxic shock syndrome and rheumatic heart disease. The latter is a particularly severe autoimmune-like disease in which cells that were originally produced to fight off the infection go on to affect heart tissue. This has posed a challenge when it came to vaccine development in the past.
“You just can’t take the whole organism and deliver it as a vaccine, because it still contains those regions that induce these cells across reactive heart tissues,” said Dr Michael Batzloff, research leader at the Institute for Glycomics. “So the focus really has been on trying to define parts of the bacteria that you can use in the vaccine that induce protective immune responses without inducing immune responses that induce pathology, or these autoimmune-like diseases.”
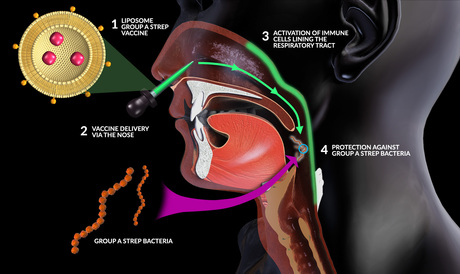
Now, the Griffith scientists have achieved exactly that with the development of their Group A Streptococcus (GAS) vaccine technology — a mucosal vaccine that is administered through the nose. The vaccine was invented by Professor Michael Good and Dr Mehfuz Zaman, with the latter noting that needle-free vaccines are economically advantageous and can aid in patient compliance.
“Essentially, it is a man-made lipid ball, composed of material that is naturally found in our own cells,” Dr Zaman said of the vaccine. “What this means is that not only is the technology well defined and well characterised, it is safe to use in humans.
“Our proprietary technology embeds the surface of these lipid balls with a small portion from the bacteria — that is, strep A — and inside the lipid ball you have a component that provides help for the immune response.”
Professor Good added that the vaccine has “enormous potential to broadly impact human health”, with the potential to prevent “a wide variety of potentially life-threatening complications and diseases in humans worldwide”.
Dr Chris Davis, general manager at the Institute for Glycomics, was responsible for negotiating the partnership with Olymvax — a major player in the emerging Chinese vaccine market. He explained, “Olymvax have commercialisation rights in China and other local jurisdictions, while Griffith University has reserved the right to commercialise the vaccine in Australia and other parts of the world.”
The licensing deal will see Olymvax conducting a Phase 1 trial in China, while the Griffith scientists will conduct their own clinical trial in Australian Indigenous populations — members of whom are particularly vulnerable to rheumatic heart disease. Pending the outcome of these trials, Griffith and Olymvax will work together to accelerate the vaccine’s development, with Dr Davis anticipating that a marketable product could be ready within 6–8 years.
How a common gene mutation increases liver disease risk
Liver damage can be caused in people after exposure to high levels of acrolein, especially in...
Gene therapy slows Huntington's disease progression in trial
Patients receiving the treatment were found to experience 75% less progression of the disease...
AI-driven manufacturing: lessons from the life sciences industry
The use of artificial intelligence for batch monitoring and digital twin development is...







