New biosynthesis method for producing antibiotics
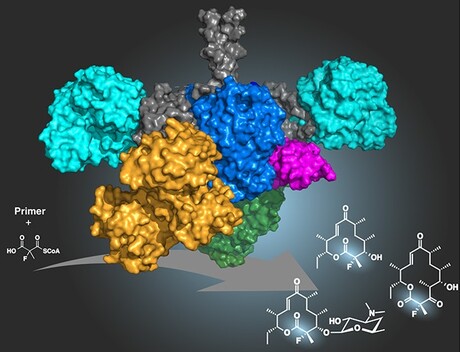
The use of the element fluorine to modify active substances is an important tool in modern drug development. German and US researchers have now successfully fluorinated a natural antibiotic via targeted bioengineering — a method through which an entire substance class of medically relevant natural products can be modified. Their work has been published in the journal Nature Chemistry.
Active drug agents have been chemically modified with fluorine for decades, owing to its numerous therapeutic effects: fluorine can strengthen the bonding of the active agent to the target molecule, making it more accessible to the body and altering the time it spends in the body. Nearly half of the small-molecule drugs (molecules up to ~100 atoms) currently approved by the US FDA contain at least one chemically bound fluorine atom, including cholesterol-lowering agents, antidepressants, anticancer agents and antibiotics.
Bacteria and fungi often manufacture complex natural compounds to obtain a growth advantage. One possible route for the development of drugs from natural compounds is to modify these substances by adding one or more fluorine atoms. In the case of the antibiotic erythromycin, for example, the attached fluorine atom confers important advantages.
The new erythromycin manufactured via this process can be accessed more easily by the body and is more effective against pathogenic microorganisms that have developed resistance to this antibiotic. However, the synthetic-chemical methods for inserting fluorine into natural substances are very complicated. Owing to the chemical and reaction conditions that are necessary, these methods are frequently “brutal”, said Professor Martin Grininger from Goethe University Frankfurt. “This means, for example, that we are very limited in selecting the positions where the fluorine atom can be attached,” he said.
Grininger and colleagues have now succeeded in utilising the biosynthesis of an antibiotic-producing bacteria. In this process, the fluorine atom is incorporated as part of a small substrate during the biological synthesis of a macrolide antibiotic.
“We introduce the fluorinated unit during the natural manufacturing process, an approach that is both effective and elegant,” Grininger said. “This gives us great flexibility when positioning the fluorine in the natural substance — and allows us to influence its efficacy.”
To this end, project leaders Dr Alexander Rittner and Dr Mirko Joppe — both members of Grininger’s research group — inserted a subunit of an enzyme called fatty acid synthase into the bacterial protein. The enzyme is naturally involved in the biosynthesis of fats and fatty acids in mice. The fatty acid synthase is not very selective in processing the precursors, which are also important for the manufacture of antibiotics in bacteria, Rittner explained. With an intelligent product design, the team succeeded in integrating a subunit of the murine enzyme into the corresponding biosynthetic process for the antibiotic.
“The exciting part is that, with erythromycin, we were able to fluorinate a representative of a gigantic substance class, the so-called polyketides,” Rittner said. “There are about 10,000 known polyketides, many of which are used as natural medicines — for example, as antibiotics, immunosuppressives or cancer drugs. Our new method thus possesses a huge potential for the chemical optimisation of this group of natural substances in the antibiotics primarily to overcome antibiotic resistance.” To exploit this potential, Rittner founded the startup kez.biosolutions to bring the team’s research results to the applications stage.
“Our technology can be used to generate new antibiotics simply and quickly and now offers ideal contact points for projects with industrial partners,” Joppe added.
Grininger has been conducting research on the tailor-made biosynthesis of polyketides for several years, and said his team’s success in fluorinating macrolide antibiotics was “a breakthrough we worked hard to achieve” as well as “an impetus for the future”.
“We are already testing the antibiotic effect of various fluorinated erythromycin compounds and additional fluorinated polyketides,” he said. “We intend to expand this new technology to include additional fluorine motifs in collaboration with Professor David Sherman and his team at the University of Michigan in the US.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Colossal announces 'de-extinction' of the dire wolf
Colossal Biosciences has announced what it describes as the rebirth of the dire wolf, which would...
Aspirin could prevent some cancers from spreading
The research could lead to the targeted use of aspirin to prevent the spread of susceptible types...
Heat-induced heart disease is killing Australians
Hot weather is responsible for an average of almost 50,000 years of healthy life lost to...




