Parkinson's progress halted in study patients
Shares in Living Cell Technologies (ASX:LCT) leapt over 40% today after the biotechnology company revealed that it had halted the progression of Parkinson’s disease in four study participants.
LCT began a Phase I/IIa clinical study of its regenerative cell therapy NTCELL in four Parkinson’s patients one year ago. The therapy functions as a neurochemical factory within the brain, producing cerebrospinal fluid (CSF) and secreting nerve growth factors that promote new central nervous system (CNS) growth and repair disease-induced nerve degeneration.
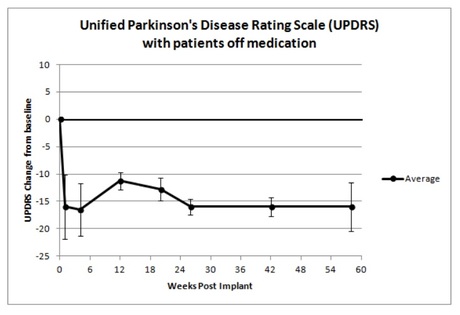
In June 2015, the company announced that the study had met its primary endpoint of safety and also improved the clinical features of Parkinson’s disease in the four patients studied. Now, 58 weeks post-implant, the treatment has stopped the progression of Parkinson’s disease and resulted in a clinically and statistically significant improvement in all the patients’ neurological scores.
Parkinson’s disease progression is measured by a neurological rating scale, Unified Parkinson’s Disease Rating Scale (UPDRS), which increases by 4–5 points each year as the disease progresses. NTCELL has successfully decreased UPDRS in the patients by an average of 16 points after 58 weeks, representing a 3–4 year reversal of neurological deterioration. In the first patient, the improvement has been maintained at 74 weeks after NTCELL implant.
LCT CEO Dr Ken Taylor said the company is “delighted with the continued positive outcome of the study”, noting that “no other treatment has been able to maintain long-term reversal of the effects of Parkinson’s disease”.
“It certainly adds anticipation and motivation to our authorised Phase IIb study,” Dr Taylor added. The planned study aims to confirm the most effective dose of NTCELL, define any placebo component of the response and further identify the initial target Parkinson’s disease patient subgroup.
Dr Taylor said LCT plans to initiate its Phase IIb study on 24 February 2016, with the ultimate goal of launching NTCELL as the first disease-modifying treatment for Parkinson’s disease in 2017.
Living Cell Technologies (ASX:LCT) shares were trading 43.90% higher at $0.059 as of around 12.30 pm on Wednesday.
Breakthrough drug prevents long COVID symptoms in mice
Mice treated with the antiviral compound were protected from long-term brain and lung dysfunction...
Antibiotics hinder vaccine response in infants
Infants who received antibiotics in the first few weeks of life had significantly lower levels of...
Colossal announces 'de-extinction' of the dire wolf
Colossal Biosciences has announced what it describes as the rebirth of the dire wolf, which would...




