Studying immune response at a single-cell level
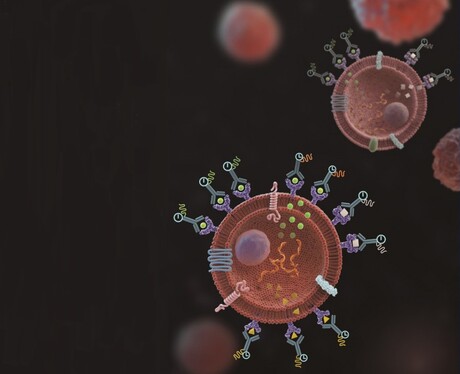
Researchers from the National University of Singapore (NUS) have invented a powerful new tool to gather data on immune response at single-cell level, which could accelerate the discovery of new immunotherapies to treat diseases such as cancer, autoimmune disorders and infectious diseases. Their work has been described in the journal Nature Methods.
Cells interact with their surrounding environment by secreting proteins which act as messengers or signals for communicating with other cells. Capturing these elusive and minute secreted proteins, particularly those from our immune cells, and correlating them to the individual source cells can provide important insights into immune responses in patients with chronic diseases such as cancer, autoimmune disorders or infectious diseases, and accelerate the development of immunotherapies.
But studying how unhealthy or healthy cells communicate, interact and coordinate with each other, in response to stimuli or pathogens, remains a challenge for scientists. To help fill the gap in correlating cell functions with their secreted proteins, the NUS researchers invented a technique called time-resolved assessment of single-cell protein secretion with sequencing (TRAPS-seq) to facilitate the studying of immune cell response at a single-cell level.
“Having progressive snapshots of the dynamic mechanism of immune cells — from responding to a viral infection to eliminating the threat — can accelerate the development of new therapeutic strategies for disease treatment, such as targeting mechanisms that are selective for cancer cells and hence spare healthy cells and reduce side effects,” said research leader Assistant Professor Cheow Lih Feng. “In addition, a better understanding of how healthy cells communicate can provide valuable insights into the normal cellular processes disrupted in cancer.”
Gathering data on cell functionality
TRAPS-seq employs surface proteins on a cell to anchor the secreted proteins by the cell, which can then be easily analysed by currently available techniques. The unique advantage of this method over other existing techniques is its capability to track the source cell secreting the proteins and measure the changes in the secretion over time when it responds to a stimulus.
“By modifying the surface proteins on a cell, we can use them as a hook to anchor a secreted protein as soon as it emerges from the cell surface,” said lead author Dr Wu Tongjin. “Using this approach, we can combine with other techniques, such as 10x Genomics’ barcoding assay, to correlate the cell’s functionality directly with its transcription and surface phenotypic profiles.”
To test the technique’s viability, the NUS team used TRAPS-seq to simultaneously monitor three types of secreted cytokine proteins from T cells, white blood cells linked to our immune defence system to fight against various diseases, by exposing them to a stimulus. The team was able to track the dynamic changes of these secreted proteins over time and observe sub-populations of T cells that show different dynamic patterns and protein secretion profiles, indicating the potential of using the method to discover new dynamic cellular mechanisms.
“TRAPS-seq provides the opportunity to measure the protein secretion profile of individual cells and resolve their functional differences,” Cheow said. “Under an abnormal condition, changes to protein secretion would have broad-ranging consequences to our immune system, tissue development and disease progression. The new technique holds great potential as a powerful tool for scientists to discover therapeutic targets for diseases relating to dysregulated protein secretion.”
Next steps
Currently, the NUS team can profile three secreted proteins at a time, but the researchers are working towards increasing the number of secreted proteins being studied simultaneously to 10.
The TRAPS-seq assay works for any secreted protein, although immediate applications are likely in immunology profiling. The researchers are optimistic that the extensive catalogues of verified antibody pairs for enzyme-linked immunoassay (ELISA) could be adapted to TRAPS-seq to detect a broad range of secreted proteins for various applications.
Antibiotics hinder vaccine response in infants
Infants who received antibiotics in the first few weeks of life had significantly lower levels of...
Colossal announces 'de-extinction' of the dire wolf
Colossal Biosciences has announced what it describes as the rebirth of the dire wolf, which would...
Aspirin could prevent some cancers from spreading
The research could lead to the targeted use of aspirin to prevent the spread of susceptible types...




