Three-person IVF: mitochondrial donation approved in UK
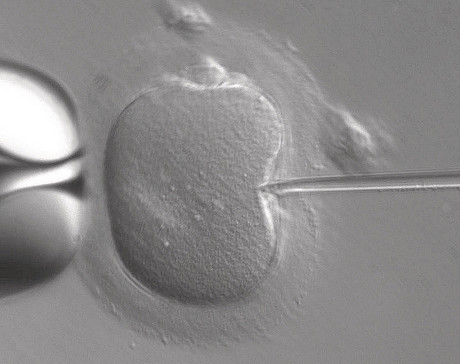
The United Kingdom has become the first country to officially authorise the clinical use of mitochondrial donation IVF to enable healthy babies to be born to women carrying deadly mitochondrial disease (mito), which starves major organs of energy. The ability to walk, run or even just stand up unaided can be a daily struggle for people with mito, which has few treatments and no cure.
The Human Fertilisation and Embryology Authority (HFEA) met in London yesterday to consider recommendations from an expert panel to license so-called ‘three-person baby IVF’, in which a future baby’s mitochondrial DNA comes from a third party. The panel recommended that the technique could be used cautiously for risk reduction treatments in certain cases where alternative treatments would be of little or no benefit to mothers at risk of passing mitochondrial disease onto their children.
“The report to the HFEA concludes that recent scientific advances have sufficiently addressed the potential carryover of faulty mitochondrial DNA,” said Professor David Thorburn, head of mitochondrial research at Murdoch Childrens Research Institute, who made a submission to the independent panel. “It also recommends numerous safeguards such as carefully selecting women to undergo the procedure as a clinical risk reduction treatment, providing full information about potential limitations and risk, and undertaking genetic testing when the embryo is at 15 weeks’ gestation.
“It is important to note that the donor mitochondrial DNA only replaces 37 mtDNA genes — contributing about 0.1% of the baby’s genetic make-up — compared with approximately 20,000 genes in the nucleus, which are not replaced.”
Parliament passed regulations permitting maternal spindle transfer (MST) and pronuclear transfer (PNT) in February 2015, and the regulatory framework has been in place since October 2015, but clinics had been advised to wait until after the HFEA had considered the panel’s recommendations before applying for permission to offer mitochondrial donation to patients. The HFEA has now approved such donation in certain, specific cases, meaning that specialist IVF clinics wanting to MST or PNT to patients may apply to the authority for permission to do so.
“After a lot of hard work and invaluable advice from the expert panel, who reviewed the development, safety and efficacy of these techniques over five years and four reports, we feel now is the right time to carefully introduce this new treatment in the limited circumstances recommended by the panel,” said HFEA Chair Sally Cheshire.
With the treatment now approved in the UK, the Australian Mitochondrial Disease Foundation (AMDF) is calling on the Australian Government to follow suit.
“At least 60 Australian babies born each year suffer with severe and life-threatening forms of mitochondrial disease that could be prevented by using the mother’s and father’s nuclear DNA and replacing the mother’s defective mitochondrial DNA with healthy mitochondrial DNA from a donor egg,” said AMDF CEO Sean Murray.
“Based on the extensive evidence available, the Australian Mitochondrial Disease Foundation believes the potential benefits of mitochondrial replacement outweigh the risks for unborn children who would otherwise almost certainly develop potentially fatal mitochondrial disease.”
Mini lung organoids could help test new treatments
Scientists have developed a simple method for automated the manufacturing of lung organoids...
Clogged 'drains' in the brain an early sign of Alzheimer’s
'Drains' in the brain, responsible for clearing toxic waste in the organ, tend to get...
World's oldest known RNA extracted from woolly mammoth
The RNA sequences are understood to be the oldest ever recovered, coming from mammoth tissue...





