Improved sterilisation of medical devices
Tuesday, 15 September, 2015 | Supplied by: Onboard Solutions

ONBoard Solutions has announced the launch of a product for the room-temperature sterilisation of tissue-engineered and medical device products: REVOX. Developed in the United States by Cantel Medical, REVOX offers a solution to what is a typically difficult and complex procedure.
The CEO of US cranial implant manufacturer OsteoSymbionics, Dorothy Baunach, described the sterilisation technology as “a lifesaver for our company". The Cleveland-based company, a designer and manufacturer of high-quality patient-specific craniofacial implants, had previously been facing a huge dilemma.
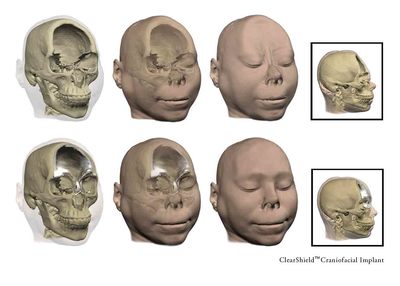
Before surgeons could use OsteoSymbionics' implants in surgery, the devices first had to be sterilised. For years, hospitals had relied on an on-site sterilisation process utilising ethylene oxide (EO) to accomplish that task. But when the chemical was recognised as a known carcinogen back in 1994, hospitals either significantly reduced their reliance on the EO process or discontinued the use of EO sterilisers completely, due to the residual risks of working with, and disposing of, such a dangerous chemical.
Without finding another reliable sterilisation method, it was getting increasingly difficult for the company to get its implants to patients in a timely manner. This was critical because patients often have a compressed time window for surgery, so alternative, off-site sterilisation methods with long lead times just wouldn't work.
Then, in early 2013, OsteoSymbionics heard about Cantel Medical's REVOX Sterilization Solutions process, claimed to be the first new sterilisation technology in the medical industry in nearly two decades. REVOX sterilisation uses a peracetic acid (PAA)-based vaporised sterilant to perform sterilisation at room temperature, between 18 and 30°C.

The REVOX sterilisation method addressed OsteoSymbionics' specific needs. Because the implants are heat sensitive, they could not be sterilised utilising EO. Chemical processes were not an option either, as the product could not retain its physical or biocompatible characteristics with such sterilisation methods. “That's what made the REVOX method such an attractive option for the company," said Baunach.
In May 2014, OsteoSymbionics was granted FDA 510(k) clearance for sterilisation using the novel REVOX Sterilisation Process.
What most appealed to Baunach and her team was that the REVOX sterilisation process enabled her company to deliver its implant to hospitals pre-sterilised, rather than relying on the hospital to sterilise. The goal, said Baunach, is to eventually have a REVOX sterilisation chamber installed and thereby speed up inline processing of the implants.
“It's nice once again to focus on what our company does best and have peace of mind that the sterilisation part of the equation is being handled so professionally," said Baunach.

Mason Schwartz, REVOX operations manager and co-inventor, said other experiments similar to their work with OsteoSymbionics are underway in the fields of donor tissue processing, porcine valves and biological — anywhere “sterility versus viability" are long-standing balancing acts. Because REVOX acts at much lower temperatures than other sterilisation technologies, Schwartz points out that it provides manufacturers with much greater flexibility when it comes to material compatibility. REVOX Sterilisation Solutions is positioned to foster increased innovation and production efficiencies for manufacturers of advanced devices.
ONBoard Solutions (a member of AusBiotech) and Cantel Medical will be presenting the REVOX Sterilisation Process at the AusBiotech Conference 2015 in Melbourne and the 2015 Annual Tissue Bank Scientific Conference in Brisbane in October. For more information on the REVOX Sterilisation Process, contact ONBoard Solutions by calling (02) 9695 1030 or emailing info@onboardsolutions.com.
Phone: 02 9695 1030
OGT SureSeq CLL + CNV V3 Panel
The updated NGS panel offers users more comprehensive variant detection to improve confidence in...
Beckman Coulter Life Sciences Cydem VT Automated Clone Screening System
The system is designed to reduce time to market for biologic drug discovery and monoclonal...
Molecular Devices CellXpress.ai Automated Cell Culture System
The cell culture system provides workflow repeatability through automation and applies...




