Blocking iron supply could prevent tuberculosis
All living organisms, including pathogens, need iron to survive. Now, researchers at the University of Zurich (UZH) have detailed the structure of the protein responsible for transporting iron to the bacteria that causes tuberculosis — and in the process identified a strategy to inhibit this protein. Their work has been published in the journal Nature.
Mycobacterium tuberculosis is a devastating pathogen and the cause of an infectious disease that generally affects the lungs. The rise of multidrug-resistant M. tuberculosis strains, which are resistant to many of the most effective anti-tuberculosis drugs, is particularly worrying. In other words, novel drugs to treat tuberculosis are urgently needed.
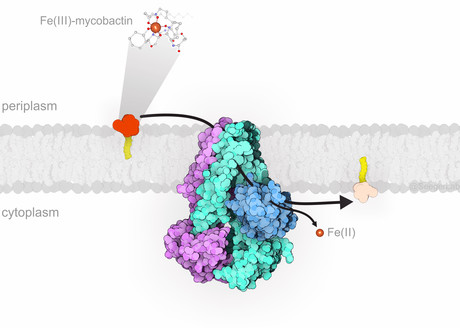
When a human cell is infected by pathogens like M. tuberculosis, it reduces iron concentration to a minimum and thereby tries to starve the invader. The tuberculosis bacteria, in turn, start to release small molecules called mycobactins. These can bind free iron extremely well and thus steal it from the host cell. The iron captured by mycobactin is then transported into the bacteria by a protein named IrtAB.
Researchers led by Markus Seeger, Professor at the Institute of Medical Microbiology at UZH, have now analysed in detail the protein responsible for transporting iron from the infected host cell into the bacteria. Prof Seeger explained, “The transport protein, which is located in the bacterial membrane, is essential for the survival of the pathogens. If IrtAB is absent or not functioning, M. tuberculosis can no longer reproduce inside the human cell.”
Using a combination of cryo-electron microscopy and X-ray crystallography, the researchers solved for the first time a high-resolution structure of the transport protein IrtAB. According to its spatial structure, IrtAB belongs to the so-called ABC exporters, which are typically involved in the efflux of molecules out of the bacterial cell.
“However, we were able to show that IrtAB in fact imports mycobactins into M. tuberculosis,” Prof Seeger said. “It therefore transports molecules in the opposite direction than expected.”
Together with scientists from The University of Texas at Dallas, the research team identified an additional peculiarity of IrtAB: it can modify the iron bound to mycobactin after it is imported into the bacteria. The iron is thus released inside the cell and the empty mycobactin can be recycled.
Seeger concluded, “IrtAB is a potential drug target, because its deletion renders M. tuberculosis inactive and incapable of infection. With our structural and functional elucidation of IrtAB, we opened avenues to develop novel tuberculosis drugs that inhibit the iron transport into the bacteria.
“In view of COVID-19, a disease that also affects the lungs, tuberculosis will likely play a more important role again in the future. It is quite conceivable that patients weakened by COVID-19 will show increased infection rates with tuberculosis.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
SEQ koala population carries immunity to retrovirus
Koalas from a population north of the Brisbane River appear to have evolved a unique genomic...
RSV immunisation program for babies slashes hospital stays
An Australian-first study has demonstrated the effectiveness of immunisation against respiratory...
A targeted treatment option for psoriasis
New research from MedUni Vienna paves the way for the development of a therapy that not only...




