COVID-19 DNA vaccine trial commences Down Under
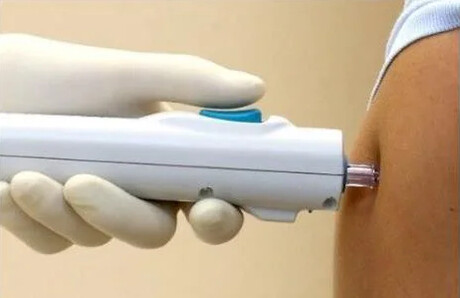
Researchers are set to commence the first Phase 1 human trial of a COVID-19 gene-based DNA vaccine in Australia, delivered via a needle-free system.
Led by The University of Sydney, Scientia Clinical Research (Sydney), Telethon Kids Institute (Perth) and the Women’s and Children’s Hospital (Adelaide), the COVALIA trial uses a gene-based vaccine with DNA sequences from the SARS-CoV-2 virus. The vaccine was developed by Australian biotech company Technovalia and its international vaccine partner, BioNet, and uses similar technology to other genetic vaccines, like mRNA, in use in Australia and internationally.
Gene-based vaccines use genetic (DNA) sequences from the virus. Researchers identify and isolate parts (genes) of the virus genome. Once the DNA is inside the cell the body uses the DNA code to make the coronavirus spike protein trigger an immune response.
The potential next-generation vaccine has no additives or preservatives. It will be given using a needle-free device that penetrates the skin with a jet spray and is designed to make sure the vaccine gets inside the cells to encourage good uptake by the immune system. While not approved outside research studies in Australia, the needle-free device is already being used to give influenza vaccines in the United States.
The Phase 1 trial will recruit 150 participants, with screening and enrolments now open. The key goal is to examine the safety of two doses of the vaccine given one month apart; if successful, a larger Phase 2 trial will be undertaken. The trial will also look at whether a lower vaccine dose may work better following vaccination into the skin rather than muscle, which is important as it could enable more people to be vaccinated with the available vaccine supply.
“We are very excited to start enrolment along with our colleagues in Perth and Adelaide, and to undertake the first needle-free COVID vaccine trial in Australia,” said lead investigator Associate Professor Nicholas Wood from The University of Sydney. “The start of the COVALIA study is a significant milestone for all involved in this one-of-a-kind partnership between Australian institutions, the industry and the Australian Government, with $3 million in funding from the Medical Research Future Fund (MRFF).”
Co-investigator Professor Peter Richmond, from the Telethon Kids Institute, added, “It is really important that we continue to develop the next generation of COVID-19 vaccines, as we may see even better safety and immune responses. Having a greater range of vaccines available increases global vaccine capacity to ensure everyone has access to immunisation.”
If you are interested in participating in the trial, visit http://www.scientiaclinicalresearch.com.au/covid-19-study/.
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Anti-inflammatory drug may help treat alcohol use disorder
A drug that is already FDA-approved for treating inflammatory conditions may help reduce both...
Osteoarthritis study uncovers new genetic links, drug targets
The genome-wide association study (GWAS) uncovered over 900 genetic associations, more than 500...
How brain cells are affected by Tourette syndrome
US researchers have conducted a cell-by-cell analysis of brain tissue from individuals with...





