Genetic cause found for rare neurological disease
The progressive neurological disease known as spinocerebellar ataxia 4 (SCA4) is a rare movement disorder whose symptoms include muscle weakness (atrophy), difficulty coordinating body movements and difficulty speaking. There is no known cure for the condition and, until recently, there was no known cause.
Now, a multinational study led by The University of Utah has conclusively identified the genetic difference that causes SCA4, bringing answers to families and opening the door to future treatments. Their results have been published in the journal Nature Genetics.
SCA4’s pattern of inheritance had long made it clear that the disease was genetic, and previous research had located the gene responsible to a specific region of one chromosome. But that region proved difficult for researchers to analyse as it was full of repeated segments that look like parts of other chromosomes, and with an unusual chemical make-up that makes most genetic tests fail.
To pinpoint the change that causes SCA4, the research team applied advanced sequencing technology to the DNA of several Utah-based SCA4 patients and their families. Family records were complete enough that the researchers were able to trace the origins of the disease in Utah back through history to a pioneer couple who moved to Salt Lake Valley in the 1840s.
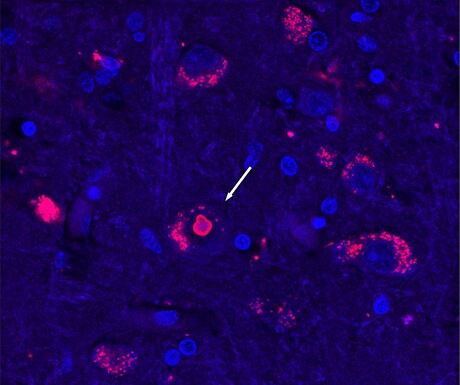
By comparing DNA from affected and unaffected individuals, the team found that in SCA4 patients, a section in a gene called ZFHX3 is much longer than it should be, containing an extra-long string of repetitive DNA. Isolated human cells that have the extra-long version of ZFHX3 show signs of being sick — they don’t seem able to recycle proteins as well as they should, and some of them contain clumps of stuck-together protein.
“This mutation is a toxic expanded repeat and we think that it actually jams up how a cell deals with unfolded or misfolded proteins,” said Professor Stefan Pulst, who co-led the study.
Healthy cells need to constantly break down non-functional proteins. Using cells from SCA4 patients, the group showed that the SCA4-causing mutation gums up the works of cells’ protein-recycling machinery in a way that could poison nerve cells.
Intriguingly, something similar seems to be happening in another form of ataxia, SCA2, which also interferes with protein recycling. The researchers are currently testing a potential therapy for SCA2 in clinical trials, and the similarities between the two conditions raise the possibility that the treatment might benefit patients with SCA4 as well.
Finding the genetic change that leads to SCA4 is essential for developing better treatments, with Pulst stating, “The only step to really improve the life of patients with inherited disease is to find out what the primary cause is. We now can attack the effects of this mutation potentially at multiple levels.”
Furthermore, simply knowing the cause of the disease can be incredibly valuable for families affected by SCA4, noted study co-leader K Pattie Figueroa. People in affected families can learn whether they have the disease-causing genetic change or not, which can help inform life decisions such as family planning.
“They can come and get tested and they can have an answer, for better or for worse,” Figueroa said.
ADHD may be linked with an increased risk of dementia
An adult brain affected by attention deficit hyperactivity disorder (ADHD) presents modifications...
Placebos appear to reduce PMS symptoms
Women affected by premenstrual syndrome (PMS) appear to experience less intense and debilitating...
Medicinal cannabis linked to long-term health benefits
As scientists find a way to improve the effectiveness of CBD, a separate study shows that...




