Hydrogel stem cell treatment repairs brain tissue in mice
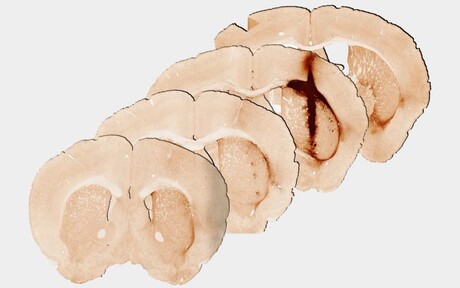
Researchers from The University of Melbourne and the Australian National University (ANU) have developed a new ‘hybrid’ hydrogel, which allows clinicians to safely deliver stem cells to the site of a brain injury in mice. Described in the journal Nature Communications, the proof-of-concept breakthrough solves a major challenge faced by stem cell researchers since the 1980s — keeping stem cells alive for long enough to allow them to evolve into the cells required to create new tissue when they are inserted into a damaged part of the body.
A hydrogel is a water-based gel that can be used to deliver substances into the body and to promote the effective growth of new cells; in this case, it supplies both the stem cells and oxygen needed to keep stem cells alive during the injection process and ensures the stem cells evolve into the type of cells needed to create new tissue to repair damage. Researchers believe this advance will benefit stem cell treatments in many parts of the body.
“After an injury such as a stroke, there is a dead area in the brain, including the blood system,” said Professor David Nisbet from The University of Melbourne, who co-led the research team. “So we need a temporary blood supply to support cells until the blood system repairs. This patented hydrogel provides that.”
Over five years of research, the team discovered that a synthetic protein based on myoglobin — a natural protein found in high concentrations in the heart muscles of sperm whales and horses — added to their hydrogel provided the sustained oxygen release needed to ensure stem cells survive the delivery process and develop into the type of cells needed to repair brain tissue. Whales and other deep-diving animals are thought to have evolved high concentrations of myoglobin in their muscle tissue so they could slowly absorb as much oxygen as possible while diving, while horses are thought to have evolved higher concentrations of myoglobin so they could run over longer distances.
Melbourne’s Professor Clare Parish conducted the mouse studies and said promising results were achieved in injured brain tissue, raising the possibility for growing new tissue for future human treatment. “Analysis at 28 days after delivery of the hydrogel revealed significantly enhanced survival and growth of the new stem cells that are needed for healthy brain functioning, compared with a hydrogel without myoglobin,” Parish said.
“We observed that the new tissue could be stimulated in a similar way to healthy brain tissue, providing the first evidence of the benefits of including oxygen delivery within a hydrogel to achieve the long-term survival and integration of stem cell transplants.”
Team co-leader Professor Colin Jackson, from ANU, said the breakthrough will interest researchers and clinicians globally and is likely to lead to many revolutionary medical treatments.
“Proof of concept has now been demonstrated within the brain of mice, but the research represents a generalisable strategy for developing injectable nanomaterials for a diverse range of applications, including cell transplantation, gene and drug delivery, 3D in vitro disease models and organ-on-a-chip technology,” Jackson said.
'Low-risk' antibiotic linked to rise of dangerous superbug
A new study has challenged the long-held belief that rifaximin — commonly prescribed to...
Robotic hand helps cultivate baby corals for reef restoration
The soft robotic hand could revolutionise the delicate, labour-intensive process of cultivating...
Stem cell experiments conducted in space
Scientists are one step closer to manufacturing stem cells in space — which could speed up...




