Needle-free vaccine patch facility opens in Brisbane
Vaxxas, a clinical-stage biotechnology company commercialising a novel vaccination platform technology, has announced the opening of its new, state-of-the-art manufacturing facility in Brisbane. The custom-built, 5500 m2 Vaxxas Biomedical Facility will serve as the company’s global headquarters and support the scale-up of its operations to produce vaccines for future late-stage clinical trials and commercial products.
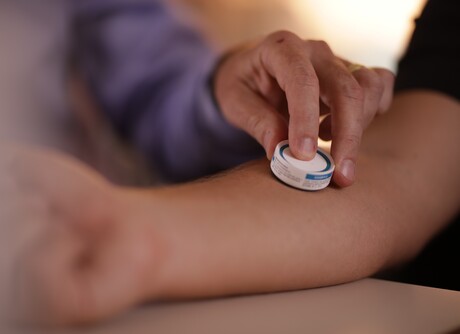
Vaxxas’s proprietary HD-MAP technology platform utilises an ultrahigh-density array of projections — invisible to the naked human eye — applied to the skin as a patch sitting inside a small applicator device. When applied to the skin, the patch delivers a vaccine to the abundant immune cells immediately below the skin surface in just seconds. In addition to being easy to use, potentially enabling self-administration, the products can be stable at room temperature, reducing the complexities and costs associated with refrigerated distribution required for many existing vaccines.
The Queensland Government provided funding and operational support to Vaxxas to facilitate the refurbishment of an existing warehouse at Brisbane’s Northshore, transforming it into a cutting-edge facility including two independent good manufacturing practice (GMP) qualified aseptic cleanrooms, a medical device manufacturing space, a device assembly cleanroom, laboratories and office space. Additional funding from the Australian Government, through its Modern Manufacturing Initiative, supported the installation of the specialised manufacturing and production infrastructure.
“This world-renowned technology has the potential to play a vital role in pandemic preparedness because it allows vaccines to be deployed quickly and easily to our communities,” said Queensland Deputy Premier Steven Miles.
“Economic Development Queensland worked closely with Vaxxas to progress designs and approval for this facility, so it’s fantastic to be here to open it.”
Vaxxas currently employs 130 people in its Queensland operations, and is planning for an increase to 200 employees over the next three to five years. The new facility will enable the company’s R&D teams to work side by side with manufacturing teams, to expand existing R&D work and streamline the translation from research to eventual commercialisation. The facility also provides a blueprint for scalable product production processes that can potentially be replicated by Vaxxas domestically and globally as demand for its platform technology grows.
Vaxxas CEO David Hoey said the facility’s opening “represents a new and exciting chapter” for the biotech company, which was founded in 2011 on research from The University of Queensland.
“The site will significantly increase our manufacturing capacity, creating new local, skilled jobs, while enabling Vaxxas to progress through late-stage clinical trials that will bring our first commercial vaccine products to the market,” he said. Manufacturing will be conducted in compliance with GMP standards, which will also meet the requirements of the Australian Therapeutic Goods Administration, the United States Food and Drug Administration, the European Medicines Agency and other global health regulators.
With several human clinical trials already completed, plus others either planned or underway, Vaxxas expects to manufacture and distribute the first commercially available needle-free vaccine patches from the Queensland facility within three to five years.
Sleep apnoea patients who use CPAP live longer: study
CPAP therapy has been found to significantly reduce the risk of death for people with obstructive...
Does exercise really extend lifespan? It's complicated
Physical activity is seen as a way to extend the human lifespan, but the benefits of physical...
Nature helps to relieve physical pain
Experiencing nature, even in the form of watching nature videos, can alleviate acute physical pain.




