Parkinson's drug induces iron deficiency, disrupts gut microbiome
Scientists from the University of Vienna, the University of Southampton, Aalborg University and Boston University have revealed that a widely prescribed Parkinson’s drug, known as entacapone, significantly disrupts the human gut microbiome by inducing iron deficiency. The team’s study, published in the journal Nature Microbiology, provides new insights into the impact of human-targeted drugs on the microbial communities that play a critical role in human health.
While it is well established that antibiotics can significantly disrupt the human gut microbiome, emerging research shows that a wide range of human-targeted drugs — particularly those used to treat neurological conditions — can also affect the microbial communities living in our bodies. Despite their intended therapeutic effects on different organs, these drugs can inadvertently disrupt the balance of gut microbes, leading to potential health consequences.
Until now, most studies investigating these interactions relied either on patient cohort analyses affected by many confounding factors or on experiments using isolated gut bacteria, which do not fully capture the complexity of the human microbiome. The research team opted to use a novel experimental approach to study the effects of two drugs — entacapone and loxapine, a medication for schizophrenia — on faecal samples from nine healthy human donors.
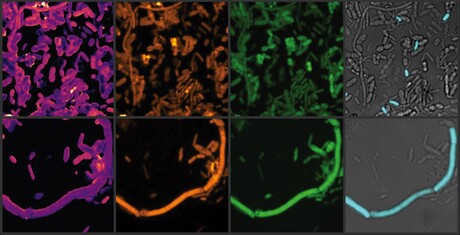
The team incubated the samples with therapeutic concentrations of the drugs, then analysed the impact on the microbial communities using advanced molecular and imaging techniques, including heavy water labelling combined with stimulated Raman spectroscopy (SRS). They discovered that loxapine and even more so entacapone severely inhibited many microbiome members, while E. coli dramatically expanded in the presence of entacapone.
“The results were even more striking when we examined microbial activity, rather than just their abundance,” said lead author Dr Fátima Pereira, formerly a postdoctoral researcher at the University of Vienna and now based at the University of Southampton. “The heavy water-SRS method allowed us to observe the subtle yet significant changes in the gut microbiome, which are often missed in traditional abundance-based measurements.”
The researchers hypothesised that entacapone might interfere with iron availability in the gut, a crucial resource for many microbes. Their experiments confirmed that adding iron to faecal samples containing entacapone counteracted the drug’s microbiome-altering effects. Further investigation revealed that E. coli, which thrived under these conditions, carried a highly efficient iron-uptake system (enterobactin siderophore). This allowed the bacteria to overcome iron starvation and proliferate, even in the presence of the drug.
“By showing that entacapone induces iron deficiency, we have uncovered a new mechanism of drug-induced gut dysbiosis, in which the drug selects for E. coli and other potentially pathogenic microbes well adapted to iron limiting conditions,” said Michael Wagner, Vice-Head of the Centre for Microbiology and Environmental Systems Science (CeMESS) at the University of Vienna.
This discovery has broader implications for understanding how other human-targeted drugs might affect the gut microbiome; several drugs, including entacapone, contain metal-binding catechol groups, suggesting that this mechanism could be a more common pathway for drug-induced microbiome alterations. The findings also present an opportunity to mitigate the side effects of drugs like entacapone, potentially by ensuring sufficient iron availability to the large intestine.
“We are looking at strategies to selectively deliver iron to the large intestine, where it can benefit the microbiome without interfering with drug absorption in the small intestine,” Wagner said.
ADHD may be linked with an increased risk of dementia
An adult brain affected by attention deficit hyperactivity disorder (ADHD) presents modifications...
Placebos appear to reduce PMS symptoms
Women affected by premenstrual syndrome (PMS) appear to experience less intense and debilitating...
Medicinal cannabis linked to long-term health benefits
As scientists find a way to improve the effectiveness of CBD, a separate study shows that...




