Putting the brakes on bacterial biofilms
A better understanding of how infectious bacteria colonise implanted medical devices could help tackle antibiotic-resistant infections that develop in biofilms.
Bacterial biofilms are made up of multicellular communities of bacteria in a self-produced matrix. They are prevalent in nature and also occur in industrial and medical settings. The active colonisation of surfaces, such as catheters implanted into the body, can lead to the spread of infection.
Biofilms are a significant threat to human and animal health because they are notoriously resistant to antibiotic treatment and immune defences. They are responsible for many chronic and recurring infections that occur around implanted medical devices.
Associate Professor Whitchurch, at the University of Technology, Sydney (UTS), leads a team of researchers from the UTS ithree institute, the UTS School of Mathematical Sciences, Monash University, the University of Melbourne and CSIRO in studying bacteria in biofilms.
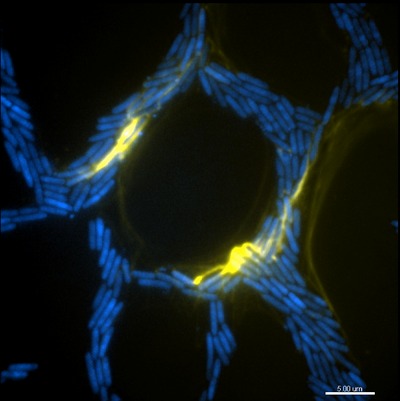
The team used high-resolution, phase-contrast time-lapse microscopy, including next-generation imaging and sophisticated computer vision analysis to develop algorithms to track and analyse how bacterial biofilms spread.
They also used atomic force microscopy to examine the topography of the substrate underneath the expanding biofilm.
Their work with the bacterium, Pseudomonas aeruginosa, a pathogen that causes respiratory disease and often causes infections when catheters are inserted, has revealed how individual cell movements are coordinated and how intricate networks of interconnected trails are formed during biofilm expansion.
“This research reveals how coordination between individual bacteria can lead to complex group behaviour to enable biofilm expansion on a surface,” said Associate Professor Whitchurch.
The researchers discovered that groups of bacteria build and migrate along a ‘furrow’ on a surface - much like a well-trodden path across a grassy field. The team also discovered that extracellular DNA (eDNA) acted not as coding molecules but as a structural ‘rope’ that helped guide the transit of the bacteria.
This ‘traffic management’ they discovered ensured a smooth flow of bacterial cells down the line towards the front of an expanding biofilm. eDNA also acted as glue, binding groups of bacteria together and forming ‘bulldozers’ that forged the furrows.
The ithree institute is pioneering the use of three-dimensional structured illumination microscopy, a super-resolution microscopy that captures real-time multiple colour images of interactions between microorganisms and living cells.
The research was published in Proceedings of the National Academy of Sciences USA.
SEQ koala population carries immunity to retrovirus
Koalas from a population north of the Brisbane River appear to have evolved a unique genomic...
RSV immunisation program for babies slashes hospital stays
An Australian-first study has demonstrated the effectiveness of immunisation against respiratory...
A targeted treatment option for psoriasis
New research from MedUni Vienna paves the way for the development of a therapy that not only...




