Researchers identify key player in cellular response to stress
An enzyme called Fic, whose biochemical role was first discovered more than a decade ago, appears to play a crucial part in guiding the cellular response to stress. The new findings, published in the journal PNAS, could eventually lead to new treatments for a variety of diseases.
Originally discovered in the Vibrio parahaemolyticus bacteria known to cause food poisoning, Fic has been a long-time focus of the Orth lab at the University of Texas Southwestern Medical Center. In 2009, Dr Kim Orth and her colleagues published the first paper showing that Fic is involved in a process called AMPylation, in which this enzyme facilitates transfer of a phosphate and adenosine group to other proteins, changing their activity. The researchers soon discovered that animals ranging from worms to humans also have a Fic enzyme.
Research in fruit flies suggested that Fic appeared to be important for stress resilience and recovery. A paper published in 2018 by Orth along with Dr Helmut Krämer and colleagues showed that flies constantly exposed to bright light, which damages their eyes, suffered permanent harm if their Fic gene was deleted through genetic engineering. However, the role of this enzyme in mammals was unclear.
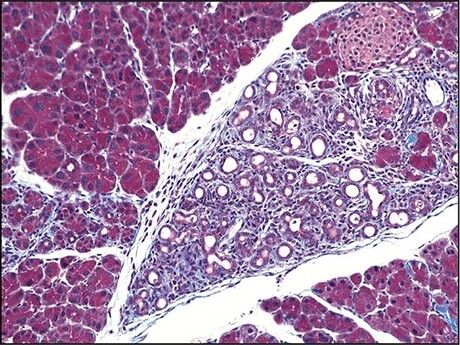
To address this issue, the researchers engineered a mouse model without a Fic gene. These animals were initially indistinguishable from littermates with Fic and appeared healthy. However, when the researchers fasted the animals for 14 hours and then allowed them to eat as much as they wanted for two hours — a stressor for the pancreas, which controls blood sugar and produces key digestive enzymes — blood work on the Fic-deficient animals showed a much higher stress response than the animals with Fic. Further investigation showed that a molecular pathway called the unfolded protein response (UPR) — which becomes activated when stressed cells can’t keep up with folding newly generated proteins — was more strongly activated in the Fic-deficient animals.
The researchers made similar findings when the mouse models were dosed with a drug called caerulein, which acts on the pancreas to force an increased output of digestive enzymes. Although animals with Fic and those without developed pancreatitis, those without this enzyme had significantly worse disease, accompanied by a significantly stronger UPR. Furthermore, although animals with Fic had a quick recovery, those without Fic developed permanent scarring in their pancreas — a sign of significantly lower resilience to stress.
“We think that Fic acts like a thermostat that adjusts a cell’s response to stressors,” said Dr Amanda Casey, a former postdoctoral fellow in the Orth lab.
Orth added that an uncontrolled cellular stress response and UPR play a role in many diseases including cancer, metabolic syndrome, atherosclerosis, retinal degeneration and various neurodegenerative disorders. She said, “If we can determine how the ‘stress thermostat’ is set, we could adjust it up or down in various diseases where stress response is a factor.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Babies of stressed mothers likely to get their teeth earlier
Maternal stress during pregnancy can speed up the timing of teeth eruption, which may be an early...
Customised immune cells used to fight brain cancer
Researchers have developed CAR-T cells — ie, genetically modified immune cells manufactured...
Elevated blood protein levels predict mortality
Proteins that play key roles in the development of diseases such as cancer and inflammation may...





