The early embryo is more in control than we thought
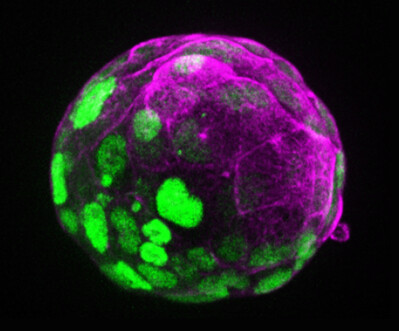
It is well known that the placenta and the uterus nurture and shelter the foetus, but the situation at the very beginning of development — when the early embryo still floats in the uterus — has been unclear so far. Now, researchers led by Nicolas Rivron at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA) have uncovered basic principles of early development using blastoids, publishing their results in the journal Cell Stem Cell.
Blastoids are in vitro models of the blastocyst, the mammalian embryo in the first few days following fertilisation. These embryo models were first developed by the Rivron lab from mouse stem cells and later from human stem cells. Blastoids can provide an ethical alternative to the use of embryos for research and, importantly, enable significant discoveries.
Using mouse blastoids, the researchers found that the early embryonic part (~10 cells) instructs the future placental part (~100 cells) to form, and the uterine tissues to change. “By doing this,” Rivron said, “the embryo invests in its own future: it promotes the formation of the tissues that will soon take care of its development. The embryo is in control, instructing the creation of a supporting surrounding.”
Indeed, the team discovered several molecules secreted by the few cells from which the foetus develops, known as the epiblast. They observed that these molecules tell other cells, the trophoblasts that later form the placenta, to self-renew and proliferate — two stem cell properties that are essential for the placenta to grow. The team also found that these molecules induce the trophoblasts to secrete two other molecules, WNT6 and WNT7B, which tell the uterus to wrap around the blastocyst.
“Other researchers had previously seen that WNT molecules are involved in the uterine reaction,” Rivron said. “Now we show that these signals are WNT6/7B and that they are produced by the blastocyst trophoblasts to notify the uterus to react. The relevance could be high because we have verified that these two molecules are also expressed by the trophoblasts of the human blastocyst.”
The team made their findings partly by examining the extent of implantation of the mouse blastoids in an in vivo implantation mouse model. Co-first author Jinwoo Seong, who performed the experiments, said, “I was very surprised by the efficiency at which our blastoids implanted into the uterus. And by changing the properties of the trophoblasts within blastoids, including the secretion levels of WNT6/7B, we could clearly change the size of the uterine cocoon.”
Because implantation is the bottleneck in human pregnancies — around 50% of pregnancies fail at that time — and WNT6 and WNT7B are also present in human blastocysts, the study findings might explain why, sometimes, things go wrong.
“We are currently repeating these experiments with human blastoids and uterine cells, all in a dish, to estimate the conservation of such basic principles of development,” Rivron said. “These discoveries might ultimately contribute to improving IVF procedures, developing fertility drugs and contraceptives.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
The University of Sydney formalises cervical cancer elimination partnership
The success of a cervical cancer elimination program has led to the signing of a memorandum of...
Noxopharm says paper reveals science behind its immune system platform
Clinical-stage Australian biotech company Noxopharm Limited says a Nature Immunology...
Neurosensing/neurostimulation implants session to be held on Monday
On Monday, a session at UNSW Sydney will include people who are benefiting from bioelectronics...



