Improving catalytic sustainability
Tuesday, 14 February, 2012
Industrial chemistry is set to improve from novel rare-earth metal catalysts that reduce waste and improve aromatic bond-forming reactions.
In chemistry, downsizing can have positive attributes. Reducing the number of steps and reagents in synthetic reactions, for example, enables chemists to boost their productivity while reducing their environmental footprint. This type of ‘atom economy’ could soon improve, thanks to a new rare-earth metal catalyst developed by Zhaomin Hou and colleagues at the RIKEN Advanced Science Institute, Wako, Japan. Their catalyst makes it simpler to modify aromatic carbon-hydrogen (C-H) bonds with silicon-bearing silyl ligands - a reaction step critical to pharmaceutical and materials science manufacturers alike.
Silicon, which is less electronegative than carbon or hydrogen atoms, can significantly alter the electronic characteristics of an organic molecule. Replacing the hydrogen atoms of an aromatic C-H group with silyl groups has emerged as an important strategy in industrial-scale chemical synthesis because these substituents can tune molecular reactivity, enabling construction of elaborate chemical frameworks.
Chemists normally use transition metals such as platinum or rhodium to catalyse aromatic silylation reactions. But to achieve high conversions, these catalysts need to be mixed with additional hydrogen acceptor reagents, which can generate unwanted waste products, including alkanes.
Hou and colleagues have pioneered studies into rare-earth metals, such as scandium, which have different catalytic properties to transition metals. Recently, they found that ‘half-sandwich’ scandium complexes, bonded on one side by a flat organic ring, showed unique activity and selectivity in the presence of carbon double bonds. This made investigations of unsaturated aromatic molecules a natural next step.
When the researchers mixed a methoxy–benzene compound called anisole with the half-sandwich scandium catalyst and a phenylsilane, they found that the silyl group substituted onto the aromatic ring with excellent selectivity and yields (Figure 1). Furthermore, the catalyst did not require hydrogen acceptor reagents and generated only H2 gas as a by-product. Hou notes that this reaction is highly advantageous in terms of atom economy.
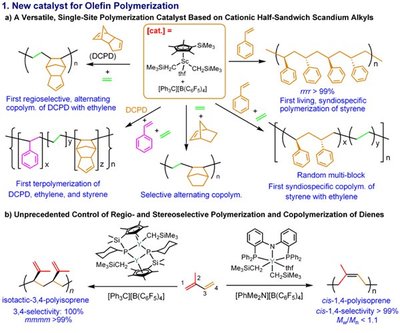
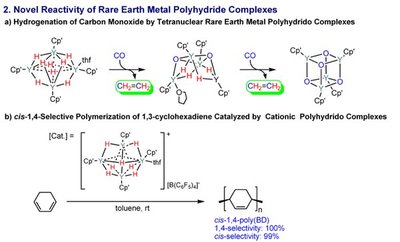
X-ray and spectroscopic measurements revealed that the working form of the catalyst, which contained a pair of ‘bridging’ hydrogen atoms, activated the reaction by coordinating the anisole’s methoxy group to the rare-earth metal. According to Hou, this relatively strong interaction directs silylation to occur almost exclusively at the position adjacent to the methoxy unit on the aromatic ring - a ‘regioselectivity’ that outshines that of transition metal catalysts, whose weak oxygen-metal interactions often produce an undesirable mix of silylation isomers.
The team will continue to explore new approaches to improving catalytic sustainability and selectivity by tapping into the extraordinary properties of rare-earth metals.
New coeliac disease test removes need for gluten consumption
The test can detect coeliac disease with up to 90% sensitivity and 97% specificity — even...
Reptiles originated 35 million years earlier than we thought
Newly discovered fossilised footprints with long toes and claws, found in a small rock slab in...
Light pollution promotes blue-green algae growth in lakes
Artificial light at night promotes the proliferation of cyanobacteria, also known as blue-green...




