Special delivery — a boron carrier for targeted tumour therapy
Japanese researchers have developed a boron carrier for use in targeted radiation treatment for cancerous tumours. The carrier is based on a common blood plasma protein, meaning it can be tailored to individual patients and lessens the chances of blood contamination.
Boron neutron capture therapy (BNCT) is a non-invasive method for the targeted destruction of cancer cells in tumours. The technique relies on the effective delivery of boron — a non-radioactive isotope — to tumours, with the patient injected with boron carriers which make their way through the bloodstream to the tumour. Once the boron has accumulated in sufficient levels in the tumour, the patient undergoes thermal neutron irradiation. The resulting miniature nuclear reaction inside the tumour blasts the cancer cells and destroys them.
Creating carriers that target tumours specifically, without discharging boron in healthy tissues, has until now been a significant challenge. So scientists from the Tokyo Institute of Technology, in collaboration with researchers across Japan, decided to test the ability of a common blood plasma protein known as albumin to carry boron to tumours during BNCT.
Albumin flows readily to and accumulates in malignant tissues; in fact, it is a major source of nutrition for growing tumours. Tumours therefore attract albumin, making the protein an ideal candidate for carrying drugs. But while it had already been used as a drug carrier for breast, lung and pancreatic cancers, its ability to transport boron for BNCT remained untested.
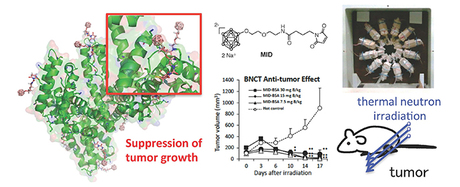
The researchers developed a tiny capsule, made from maleimide-functionalised closo-dodecaborate (MID), and trialled its ability to bind with albumin and to carry boron. They found that MID successfully binds with both cysteine and lysine residues in albumin, creating a stable carrier for boron that the team called MID-AC.
MID-AC was tested on 26 tumour-bearing mice. The team found that the boron concentrated highly and efficiently in tumours; the active uptake of boron by the albumin led to double the concentration of boron reaching tumours than previous systems. Levels of boron in the bloodstream and organs were very low, indicating a viable targeted delivery system. Thermal irradiation was carried out on the mice and led to the significant suppression of tumour growth even at low boron levels.
The researchers say their technique could limit existing problems of using blood-based products in cancer treatment. Albumin could be taken from patients in hospital prior to treatment, then used in MID-AC to carry boron to tumours in the patients’ own bodies. This will limit contamination — a risk when using blood products from other people — and encourage the acceptance of the system by the patients’ immune systems.
Additionally, albumin’s natural propensity to gather in tumours means that boron levels in surrounding healthy cells and organs is limited. This reduces the risk of side effects during radiation-based cancer therapy.
The study has been published in the Journal of Controlled Release.
Solar-powered reactor uses CO2 to make sustainable fuel
Researchers have developed a reactor that pulls carbon dioxide directly from the air and converts...
Scientists simulate the effects of an asteroid collision
How would our planet physically react to a future asteroid strike? Researchers simulated an...
2024 was warmest year on record, 1.55°C above pre-industrial level
The World Meteorological Organization says that 2024 was the warmest year on record, according to...




