Selective combustion removes pollutants from industrial processes
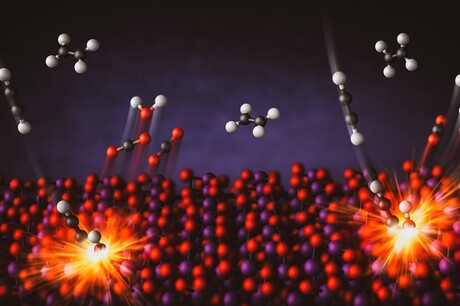
A research team led by the University of Minnesota Twin Cities has discovered how a catalyst can be used to selectively burn one molecule in a mixture of hydrocarbons, which are compounds made of hydrogen and carbon atoms. Described in the journal Science, the new method could help with the removal of pollutants and improve efficiency for industrial processes ranging from the production of fuels and medications to fertilisers and plastics.
By using a bismuth oxide catalyst — a substance that speeds up a chemical reaction — the researchers could selectively burn one molecule in a mixture of combustibles. They showed that you can effectively combust even small amounts of acetylene in mixtures with ethylene. Removing acetylene is a crucial process to prevent poisoning of polymerisation catalysts, which is vital for the production of polyethylene plastics — a market that exceeds 120 million tonnes annually.
“No one else has shown that you could combust one hydrocarbon present in low concentrations, in mixtures with others,” said Professor Aditya Bhan, lead investigator on the study.
Conventionally, combustion processes are used to burn all hydrocarbon fuel mixtures at high temperatures to produce heat. The use of a catalyst allowed the researchers to tackle the challenge of burning one molecule but not the others. The bismuth oxide catalyst is unique as it provides its own oxygen during combustion, rather than using oxygen from an outside source, in a process called chemical looping.
“We were able to take oxygen out of the catalyst and put it back in multiple times, where the catalyst changes slightly, but its reactivity is not impacted,” said PhD candidate Matthew Jacob, first author on the paper. “Operating in this chemical looping mode avoids flammability concerns.”
Traditionally, eliminating small concentrations of contaminants is very challenging and energy-intensive, but this new method could provide a more energy-efficient alternative.
“You want to do this process selectively,” said Professor Matthew Neurock, senior co-author on the paper. “Removing acetylene and other trace hydrocarbon contaminants in this manner could be more energy efficient. You just want to be able to go into a gas mixture to remove some molecules without touching the rest of the molecules.”
The researchers said the long-term impact could be high because catalysts are used in the production of just about anything we touch in modern society from fuels and medications to fertilisers and plastics. Understanding how molecules combust — and don’t combust — on catalyst surfaces is valuable for making fuels and plastics production more efficient.
“If we can understand how a catalyst works, at a molecular atomic level, we can adapt it to any particular reaction,” said study co-author Simon Bare, a Distinguished Scientist at the SLAC National Accelerator Laboratory at Stanford University. “This can help us understand how catalysts, that produce fuels and chemicals needed in modern living, react to their environment.”
Colon cancer DNA in blood can guide chemo decisions
A simple blood test could change how doctors decide which patients with colon cancer need...
Non-invasive blood test helps rule out oesophageal cancer
Designed and developed in Australia, the PromarkerEso test is designed to offer a quick,...
Taste-based flu test enables rapid diagnosis
The diagnostic tool consists of the sensor molecule thymol and a virus-specific sugar building...



