Successful phase 2 results in Huntington disease trial
Prana Biotechnology has announced the results of its phase 2 clinical trial investigating the drug PBT2 as a treatment for Huntington disease. Conducted by the Huntington Study Group at research sites in the United States and Australia, the study enrolled 109 individuals with Huntington disease who were randomly assigned to receive daily doses of either PBT2 250 mg, PBT2 100 mg or a placebo for 26 weeks.
The drug was found to be safe and well tolerated, with 95% of participants completing the study on their assigned dose. There were no substantial differences in adverse events across the groups. Only one adverse event was deemed to be related to drug treatment, and this occurred during the four-week follow-up period after completing the treatment.
The effects of PBT2 were tested on cognition, motor performance, behaviour and functional capacity, of which cognition was pre-specified as the main efficacy outcome. The 250 mg group showed improvement in performance in the Trail Making Test Part B - a measure of executive function which is impaired early in the course of Huntington disease.
An earlier trial had shown that PBT2 improved executive function in Alzheimer’s disease patients, so the latest trial included a plan to assess its effects on an Executive Function Composite z-score that included the Trail Making Test Part B. There was significant improvement in this z-score in a pre-specified analysis of participants with early-stage Huntington disease, as measured by their Total Functioning Capacity score. Across all participants, comprising both early- and mid-stage patients, there was a trend to improvement.
“The observation of significant improvement in executive function with PBT2 in this clinical trial for Huntington disease, and the previously reported Alzheimer’s trial, suggests a common mechanism for neurodegeneration in these diseases based on metal interactions,” said Dr Rudy Tanzi, Professor of Neurology at Harvard Medical School and Prana’s chief scientific advisor. “In my opinion, these findings significantly elevate the potential for PBT2 as an effective therapy for both Huntington disease and Alzheimer’s disease.”
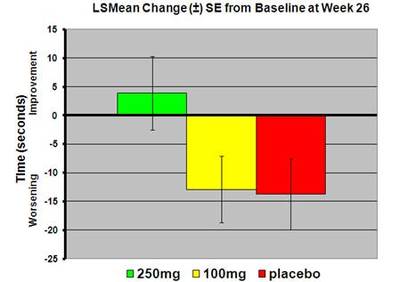
Dr Ira Shoulson, Professor of Neurology at Georgetown University and chair of the Huntington Study Group, noted, “The improvement in executive function performance was also accompanied by a favourable signal of a slowing of functional decline, as measured by the Total Functional Capacity score.
“This is the first time we have observed dose-related slowing in functional decline over a six-month period of treatment which, taken together with the safety reassurance, will provide genuine optimism for the Huntington disease community to support a larger confirmatory trial of PBT2 in Huntington disease.”
As Huntington disease and other neurodegenerative disorders progress, there is a gradual loss of brain tissue or atrophy. A subset of trial patients was given MRIs to map anatomical changes in brain structure. In the combined PBT2 groups, a reduction in atrophy of brain tissue in regions of the brain known to be affected by Huntington disease was observed compared to the placebo group.
“Despite the very small number of patients in the sub-study, the data are suggestive of a beneficial effect of PBT2 in regions of the brain that are known to be vulnerable to Huntington disease,” said Dr Diana Rosas, Associate Professor of Neurology at Harvard Medical School and the study’s co-principal investigator.
According to Dr Ray Dorsey, Professor of Neurology at the University of Rochester and the principal investigator on the trial, “The results indicated a significant benefit on cognition that is consistent with the previous trial in Alzheimer’s disease and is accompanied by an encouraging finding in functional capacity.” As a result, Prana plans to advance into a confirmatory phase 3 clinical trial that could allow PBT2 to be approved for the treatment of Huntington disease.
How light helps plants survive in harsh environments
Researchers from National Taiwan University have uncovered how light stabilises a key...
SKA-Low's first image of the universe released
The image is an indication of the scientific revelations that will be possible with the...
Which blood test is best at monitoring ALS?
A new study compares three types of blood biomarkers: neurofilament light chain proteins, glial...




