UV light separates rare-earth elements
Researchers from KU Leuven have discovered a method to separate the rare-earth elements europium and yttrium with UV light instead of traditional solvents. Their findings offer new opportunities for the recycling of fluorescent lamps and low-energy light bulbs.
Commonly used in sustainable technology and high-tech applications, europium and yttrium are difficult to mine; hence, there is great interest in recycling them. The elements can be recovered from red lamp phosphor - a powder that is used in fluorescent lamps such as neon tubes - but it can be technically complex to recuperate them from end-of-life fluorescent lamps using traditional solvents.
Earlier this year, KU Leuven chemists developed ionic liquid technology to recycle europium and yttrium from collected fluorescent lamps and low-energy light bulbs. Their method recycles the red lamp phosphor as a whole to re-use the powder in lamps. For other applications, however, it is necessary to separate europium and yttrium from the rare-earth mixture - a complicated process.
“The traditional method dissolves europium and yttrium in aqueous acid,” said Professor Tom Van Gerven, the study’s corresponding author. “An extractant and a solvent are then added to the aqueous liquid, leading to two separate layers known as ‘phases’: an aqueous layer containing the rare earth metals and a solvent layer with the extractant. When the two layers come into contact, one of the two rare earth metals is extracted to the solvent, while the other rare earth metal remains in the aqueous layer.”
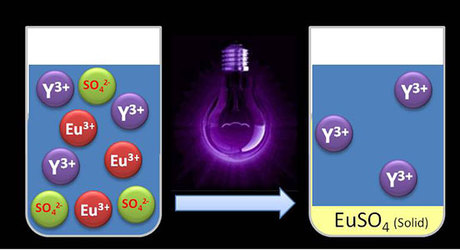
But this process needs to be repeated dozens of times to recover a high percentage of a particular rare earth metal, and there will still be traces of yttrium in the europium-containing liquid and vice versa. The KU Leuven teams have now collaborated to recover europium from the liquid mixture with UV light instead of a solvent, with the results published in the journal Green Chemistry.
“The UV light influences the electrically charged particles known as ions. Both europium and yttrium have three positive charges per ion,” said study co-author Bart Van den Bogaert, who is preparing a PhD on the subject. “When we shine UV light upon the solution of europium and yttrium, we add energy to the system. As a result, one positive charge per europium ion is neutralised. When we add sulfate, only the europium reacts with it. The result is a precipitate that can easily be filtered, while the yttrium remains in the solution.”
UV light does not leave behind any harmful chemicals in the liquid, and the separation efficiency and purity in synthetic mixtures are very high: more than 95% of the europium is recovered from the solution. The precipitate itself is 98.5% pure, so it contains hardly any traces of yttrium.
A similar purity was obtained with industrial mixtures, but the efficiency of the separation still needs to be improved. That will be one of the next projects tackled by the researchers.
Liquid catalyst could transform chemical manufacturing
A major breakthrough in liquid catalysis is transforming how essential products are made, making...
How light helps plants survive in harsh environments
Researchers from National Taiwan University have uncovered how light stabilises a key...
SKA-Low's first image of the universe released
The image is an indication of the scientific revelations that will be possible with the...




