Enhancing DNA and oligonucleotide sample preparation
The difficulties of concentration of oligonucleotides, and especially tagged oligonucleotides, are well documented. Adverse conditions can damage the sample and, in some cases, totally degrade it. When sourcing a concentration method for their microtitre plates containing oligonucleotides (oligos), researchers at high-throughput genome analysis centres must take care to choose an evaporator system that will not only provide fast drying, but also take good care of their samples.
Concentration and microarray production
At the Wellcome Trust Centre for Human Genetics (WTCHG) in the UK, the microarray team run a high-throughput custom microarray platform studying mouse and rat gene expression. For these experiments, total RNA is harvested from tissue or cells, amplified, labelled and hybridised onto oligo printed slides. The experiments show the differential expression between a control and the study sample. It is in preparing the oligo printed slides that a problem arises.
The oligo printed slides are typically prepared using 70-mer oligos stored in over thirty 384-well plates. The 384-well plates contain oligos re-suspended in a volatile phosphate buffer; the buffer causes an unknown volume of water to evaporate during the microarray printing process. This does not affect the printing process but may affect subsequent printing, as the oligos are at a higher and unknown concentration than before. The WTCHG considered that the optimum method of ensuring a known concentration is to dry the plates between each printing run and to re-suspend the oligos to a known concentration when required. The oligo plates are stored dry in between runs.
The centre had an elderly vacuum concentrator which could accept only two plates at a time and took up to 3 h to dry them. Therefore, it took two weeks to dry all the plates after each run. To preserve the integrity of the plates awaiting drying, these were frozen at -18°C and thawed when the concentrator was available. It is well documented that freeze thaw cycles can be detrimental to oligo quality and can cause degradation. The centre began to search for a new high-throughput concentrator which could keep up with their microarray production. The system they selected was the Genevac EZ-2 personal evaporator (Figure 1). The EZ-2 can take eight plates per run and dries them in just over an hour, meaning that all the plates for a microarray printing run can be dried within one day. Samples awaiting drying are kept in the fridge at 4°C, eliminating the need to freeze and preventing damage to samples.

Researchers from the centre report that they have more confidence in the quality of their oligos and believe that they are of higher quality now that they have introduced state-of-the-art concentration processes. These findings support the work of Knight, who discusses the importance of correct drying for best results within MALDI target production.
High-density microarray preparation
Another team at the centre are studying hereditable disease by analysing single-nucleotide polymorphisms (SNPs). In the procedure for the detection of the disease, the genomic DNA of the individual is hybridised to the SNP array and will bind with greater frequency with the SNPs associated with that person; the array is then visualised by fluorescence. The presence of the SNPs in the genomic DNA indicates that the person is susceptible to the disease.
In the identification of the disease threat, it is clearly imperative that the researcher has confidence in the quality of the array. This integrity of product is dependent on the way the detection array is synthesised and constructed. One of the key steps in this process is achieving a concentration of the cDNA in the array that will give a signal when visualising. The technique used during concentration of the sample may be damaging, contributing to inaccuracies and/or low yields.
To study SNPs, high-throughput researchers operate the Sequenom MassARRAY SNP Genotyping system. MassARRAY is a high-throughput, high-fidelity system for SNP analysis within genotyping studies which performs all steps of the assay and analysis in the one system. The technology employed requires very small volumes of sample, specifically 5 µL, containing as little as 2.5 ng of material. The Sequenom system is very specific in its requirements for sample preparation. To achieve the required volume of 5 µL, some samples needed to be concentrated; this used to be achieved by air drying the samples over a number of days. Using the Genevac EZ-2 personal evaporator, these samples are concentrated aseptically within the hour, saving time and reducing risk of contamination.
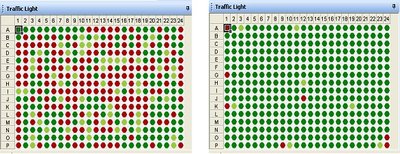
Methods using the EZ-2 evaporator were compared to the previous standard method where plates were air dried over a period of time. Plates containing identical samples were dried using each method and then analysed using the MassEXTEND reaction. The duplex pass rate for each well is represented in the traffic light diagram in Figure 2a, where dark green shows good data, light green mid-quality data and red shows poor or no data. The results of a 2-plex assay looking for two different SNPs within the same plate are shown in Figure 2b. The number of ‘no calls’, ie, no data, has significantly reduced on both assays. The quality of results achieved following drying on the EZ-2 evaporator are clearly better than achievable with simply air drying in this case.
| Genotype | Assay 1 air drying (%) | Assay 2 air drying (%) | Assay 1 EZ-2 (%) | Assay 2 EZ-2 (%) |
| G | 0.30 | 27.60 | 0.78 | 44.53 |
| T | 33.07 | 5.99 | 77.60 | 8.07 |
| GT | 7.03 | 20.83 | 19.79 | 35.68 |
| no calls | 59.64 | 45.57 | 1.82 | 11.72 |
Summary
When working with DNA and oligo samples, great care needs to be taken at every stage to ensure that degradation does not occur and the highly potent samples are not contaminated. Use of state-of-the-art concentration systems can significantly speed up concentration rates, saving researchers time, and also eliminate potential sources of damage to samples.
Novel activity identified for an existing drug
Drug discovery company Re-Pharm has used computational chemistry suite Forge, a product of its...
New structural variant of carbon made of pentagons
Researchers from the US and China have discovered a structural variant of carbon called...
Cosmic radio waves caught in real time
Swinburne University of Technology PhD student Emily Petroff has become the first person to...




