New method for fluorescence lifetime microscopy
Conventional fluorescence microscopy provides poor quantitative information of the sample because it only captures fluorescence intensity, which changes frequently and depends on external factors. Now, Japanese scientists have found a way to measure both fluorescence intensity and lifetime, publishing their new technique in the journal Science Advances.
Fluorescence microscopy is widely used in biochemistry and life sciences because it allows scientists to directly observe cells and certain compounds in and around them. Fluorescent molecules absorb light within a specific wavelength range and then re-emit it at the longer wavelength range. However, the major limitation of conventional fluorescence microscopy techniques is that the results are very difficult to evaluate quantitatively; fluorescence intensity is significantly affected by both experimental conditions and the concentration of the fluorescent substance.
A way around the conventional problem is to focus on fluorescence lifetime instead of intensity. When a fluorescent substance is irradiated with a short burst of light, the resulting fluorescence does not disappear immediately but actually ‘decays’ over time in a way that is specific to that substance. The ‘fluorescence lifetime microscopy’ technique leverages this phenomenon — which is independent of experimental conditions — to accurately quantify fluorescent molecules and changes in their environment. However, fluorescence decay is extremely fast, and ordinary cameras cannot capture it. While a single-point photodetector can be used instead, it has to be scanned throughout the sample’s area to be able to reconstruct a complete 2D picture from each measured point. This process involves movement of mechanical pieces, which limits the speed of image capture.
Now, researchers from Tokushima University have developed a novel approach to acquire fluorescence lifetime images without necessitating mechanical scanning of a focal point; instead, it produces images from all points in the sample simultaneously, enabling a more quantitative study of dynamic biological and chemical processes.
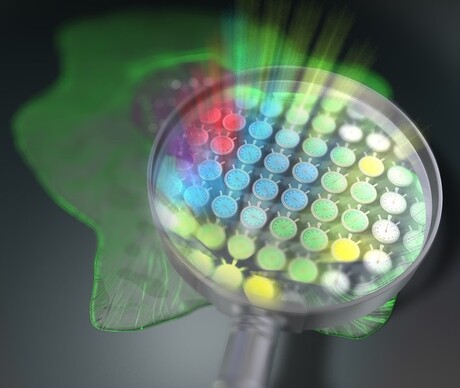
“Our method can be interpreted as simultaneously mapping 44,400 ‘light stopwatches’ over a 2D space to measure fluorescence lifetimes — all in a single shot and without scanning,” explained Professor Takeshi Yasui, who led the study.
One of the main pillars of the new method is the use of an optical frequency comb as the excitation light for the sample. An optical frequency comb is essentially a light signal composed of the sum of many discrete optical frequencies with a constant spacing in between them. The word ‘comb’ in this context refers to how the signal looks when plotted against optical frequency: a dense cluster of equidistant ‘spikes’ rising from the optical frequency axis and resembling a hair comb.
Using special optical equipment, a pair of excitation frequency comb signals is decomposed into individual optical beat signals (dual-comb optical beats) with different intensity-modulation frequencies, each carrying a single modulation frequency, and irradiated on the target sample. The key here is that each light beam hits the sample on a spatially distinct location, creating a one-to-one correspondence between each point on the 2D surface of the sample (pixel) and each modulation frequency of the dual-comb optical beats.
Because of its fluorescence properties, the sample re-emits part of the captured radiation while still preserving the aforementioned frequency–position correspondence. The fluorescence emitted from the sample is then simply focused using a lens onto a high-speed single-point photodetector. Finally, the measured signal is mathematically transformed into the frequency domain, and the fluorescence lifetime at each ‘pixel’ is easily calculated from the relative phase delay that exists between the excitation signal at that modulation frequency versus the one measured.
Due to its impressive speed and high spatial resolution, the microscopy method should make it easier to exploit the advantages of fluorescence lifetime measurements. According to Prof Yasui, “Because our technique does not require scanning, a simultaneous measurement over the entire sample is guaranteed in each shot. This will be helpful in life sciences where dynamic observations of living cells are needed.”
The study therefore showcases how optical frequency combs, which were previously only being used as ‘frequency rulers’, can find a place in microscopy techniques. In addition to providing deeper insight into biological processes, the new approach could be used for simultaneous imaging of multiple samples for antigen testing, which is already being used for the diagnosis of COVID-19. It also holds promise for the development of novel therapeutic options to treat intractable diseases and enhance life expectancy.
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Benchtop NMR used to assess heart disease risk
The breakthrough will enable more accessible, high-throughput cardiovascular disease (CVD) risk...
Activated gold helps visualise drug movement in the body
Gold nanoparticles are promising drug carriers for cancer therapy and targeted drug delivery, but...
Plasma-modified graphene enhances gas sensors
Researchers have improved gas-sensing technology by treating graphene sheets with plasma under...




