Smart nanoprobe lights up prostate cancer cells
Biomedical imaging modalities such as magnetic resonance imaging (MRI) have revolutionised the ability to detect and track the progress of many cancer types; however, the difficulty of obtaining detailed images of cancer cells buried deep within normal tissues has slowed the usefulness of such technology for personalised cancer care. Biomedical engineers are now overcoming this limitation by building nanoprobes that are designed to travel throughout the body, accumulate in cancer cells, and send a signal that illuminates the tumour.
Researchers at Johns Hopkins University, supported by the US National Institute of Biomedical Imaging and Bioengineering (NIBIB), recently developed a smart nanoprobe designed to infiltrate prostate tumours and send back a signal using an optical imaging technique known as Raman spectroscopy. The nanoprobe, described in the journal Advanced Science, has the potential to determine tumour aggressiveness and could also enable sequential monitoring of tumours during therapy to quickly determine if a treatment strategy is working.
“We are excited about the potential for improving diagnosis and treatment of many common cancers,” said project leaders Dr Jeff Bulte and Dr Ishan Barman. “We are combining self-assembling nanoprobes with Raman spectroscopy to achieve precise, single-cell resolution images required for eventual practical use in the clinic.”
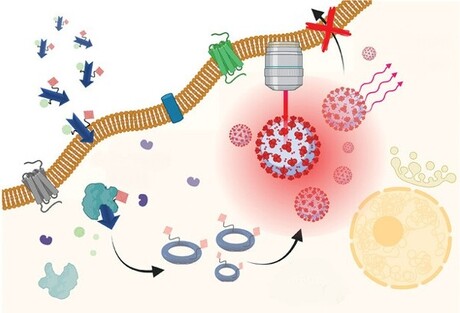
The engineering team constructed a nanoprobe that is sensitive to its local microenvironment: it is activated only after it encounters legumain, a tumour-associated enzyme that is produced by aggressive prostate cancer cells. Once it encounters the nanoprobe, legumain splits it into pieces that can self-assemble to create an optically active nanoparticle. These nanoparticles emit specific wavelengths of light that can be detected with Raman spectroscopy to visualise the tumour.
Postdoctoral fellows Dr Swati Tanwar and Dr Behnaz Ghaemi spearheaded the design and synthesis of the nanoprobe, called nanoSABER (for Self-Assembling Bioorthogonal Enzyme Recognition) — a name that reflects the surgical precision of the smart molecule.
“We have chosen Raman reporters that are specifically active in the ‘cell silent’ region of the near-infrared spectrum to avoid interference with the signal from normal tissue,” Barman said. “This selective activity is crucial for our imaging technique, as it allows for precise detection by Raman spectroscopy without reacting with or being obscured by the surrounding biological material.”
The researchers used two prostate cancer cell lines exhibiting varying levels of legumain expression — one high and one low — alongside a non-cancerous prostate epithelial cell line, which produces a negligible amount of the enzyme.
NanoSABER was tested in laboratory cell cultures and in an experimental mouse model. In both settings, the prostate cells expressing legumain activated the nanoprobe and emitted a signal with an intensity that corresponded to the amount of legumain produced by the cancer cells. The non-legumain cell type did not activate the nanoprobe, demonstrating that the nanoSABER system performed as designed, emitting a signal that correctly indicated the presence and amount of the cancer-associated enzyme.
The researchers believe they have engineered a molecular system with the potential to not only identify tumours using optical imaging, but to also rapidly assess tumour aggressiveness — potentially without the need for painful biopsies that are the current standard of care. In addition, as profiles of enzyme secretion by different types of cancers are discovered, additional nanoSABER probes can be synthesised that will allow a level of precise diagnosis of tumour types and characteristics that is not currently possible, including sequential imaging of tumours to determine whether therapies are working in real time.
“The work by this group is a significant step towards better care for men afflicted with prostate cancer,” said Dr Tatjana Atanasijevic, a program director in the Division of Applied Science & Technology at NIBIB. “It is an excellent example of the type of innovative technologies NIBIB supports that have the potential to dramatically impact health care.”
Plasma-modified graphene enhances gas sensors
Researchers have improved gas-sensing technology by treating graphene sheets with plasma under...
Laser-powered device could detect microbial fossils on Mars
The first life on Earth formed four billion years ago, as microbes living in pools and seas: but...
Life's building blocks found in Bennu asteroid sample
A new analysis of samples from the asteroid Bennu reveals that evaporated water left a briny...




