Unravelling complex biomolecular structures
Friday, 30 November, 2012
A new mass spectrometer developed by a Utretch University’s Heck Lab and Thermo Fisher collaboration is offering new insight into molecularly complex biomolecules.
The Heck Lab at Utrecht University, which is more formally known as the Biomolecular Mass Spectrometry and Proteomics Group, has collaborated with Alexander Makarov of Thermo Fisher, the inventor of the Orbitrap analyser, to develop a new mass spectrometer. This highly sensitive instrument might play a crucial role in the development and use of therapeutic antibodies.
The researchers, led by Albert Heck, have shown that protein assemblies of molecular weights over 1 million Da can be analysed with very high analytical resolving power and exquisite sensitivity down to detection of single ions. The new mass spectrometer allows the measurement of a range of important proteins and protein assemblies allowing a detailed analytical footprint of these biologically and medically important molecules. Especially in the fast-growing arena of biopharmaceuticals such as therapeutic antibodies, this new instrument will be important both in R&D and in quality control, to enable such molecularly complex biomolecules to be used safely in the clinic.
According to Heck: “The impact of the high mass resolving power at very high sensitivity as achievable with this new mass spectrometer is tremendous; it opens up avenues to measure not only protein-protein interactions, but also covalent and non-covalent binding of small molecules to protein assemblies. Wide-ranging applications may include the direct analysis of drug molecules binding to their targets, and the investigation of post-translational and chemical modifications (eg, phosphorylation, glycosylation) on intact proteins and protein assemblies. I foresee that this instrument will become instrumental in the development and use of therapeutic antibodies, but also, for instance, in analysing how drug molecules such as proteasome inhibitors do interact with their target, the proteasome.”
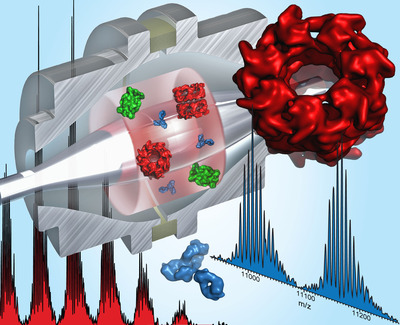
Makarov added: “Presently, Orbitrap mass spectrometry is probably the fastest growing mass spectrometric technique. Through this collaboration with Utrecht University we have opened up new avenues for the use of this mass analyser. I always believed in the versatility of the Orbitrap analyser, but am still amazed to see that we can now also mass analyse huge protein complexes, even whole viruses, with substantially improved resolving power and mass accuracy and sensitivity down to individual ions.”
This research was made possible by support of the Netherlands Proteomics Centre and the EU-funded large-scale facility PRIME-XS.
Background on the analyses of therapeutic antibodies
Since the mid-1990s, antibodies have become an important class of drugs, with more than 28 antibodies approved for therapeutic use in the US and Europe. The need to improve clinical efficacy of antibodies further is continuously ongoing. Engineering of antibodies has enabled the design of antibody-based formats with tailored pharmacokinetics, avidity, (bi-)specificity and increased tumour penetration. Modification of the N-linked glycosylation of monoclonal antibodies has also received interest as a strategy for improving the efficacy of therapeutic antibodies. Moreover, mixtures of antibodies, bi-specific antibodies and antibody drug conjugates are rapidly entering the therapeutic arena. Antibodies are large and complex glycoproteins (150,000 Da), and their complexity becomes only further enhanced by all the abovementioned new strategies. The essential detailed analytical molecular characterisation of these therapeutics poses enormous challenges to the field of analytical chemistry. The technique of mass spectrometry surfaces as the key technology for such analysis, especially when it allows analysis at very high sensitivity, accuracy, speed and selectivity.
Biosimilar antibodies
Such analysis also becomes important for the identification and analysis of biosimilar antibodies that are copy versions of the original ones that will be out of patent in the next decade. It is not possible to produce exact molecular copies of antibodies, as they are produced from different cell clones undergoing different manufacturing processes. As a consequence, micro-variations can be introduced that impact safety and potency. Only very small differences between biosimilar and reference mAbs, with reassurance that these are not of clinical relevance, may be accepted by health authorities. Copy versions of the original biopharmaceuticals are already available in several countries but no consistent worldwide requirements for their registration are established so far.
Benchtop NMR used to assess heart disease risk
The breakthrough will enable more accessible, high-throughput cardiovascular disease (CVD) risk...
Activated gold helps visualise drug movement in the body
Gold nanoparticles are promising drug carriers for cancer therapy and targeted drug delivery, but...
Plasma-modified graphene enhances gas sensors
Researchers have improved gas-sensing technology by treating graphene sheets with plasma under...




