An advance in Alzheimer's research
As a young PhD in 1996, Chilean-born molecular geneticist Mauricio Arcos-Burgos suffered the acute disappointment of running a close second to a Canadian research team in the race to publish the discovery of a key mutation that causes early-onset Alzheimer’s disease (AD).
The mutation in the Presenilin 1 (PSEN1) gene substitutes the amino acid glutamine (E) for the normal alanine (A) at residue 280 in the Presenilin 1 protein. The E280A mutation greatly accelerates deposition of the pathogenic amyloid-β protein fragment that clogs the brains of AD patients, causing progressive mass-death of nerve cells at a relatively young age.
Four years ago, Arcos-Burgos was appointed Associate Professor with the Genomics and Predictive Medicine Group of the Australian National University’s College of Medicine, Biology & Environment. He says E280A mutation behaves as a fully penetrant Mendelian dominant — every individual who inherits the mutant allele is almost guaranteed to develop early-onset AD somewhere between age 50 and 70.
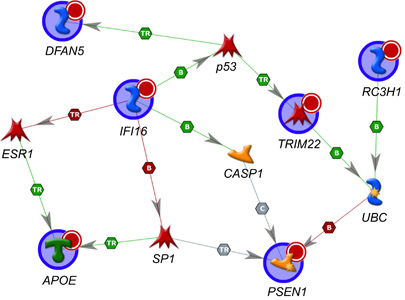
An international collaboration led by Arcos-Burgos recently announced in Molecular Psychiatry that it has identified a network of genes whose interactions influence the age at which carriers of the pathogenic E280A mutation will develop PSEN1-type Alzheimer’s disease. Arcos-Burgos and his colleagues identified the genes by screening the genomes of 71 members of the world’s best known PSEN1-type AD pedigree — Colombia’s extended Paisa family.
The Paisa family’s genetic misfortune has been traced to a Spanish conquistador who settled in northern Colombia’s mountainous Antioquia region in the early 1500s. The mutations spread in the conquistador’s 5000 latter-day descendants provide a textbook example of genetics’ founder effect.
Two decades ago, genetic linkage analyses of affected and unaffected Paisa family members allowed Arcos-Burgos and his Canadian rivals to locate the pathogenic mutation to the PSEN1 gene on human chromosome 14. Among the genes identified in the latest study, some speed the onset of PSEN1-type Alzheimer’s disease. But some have a beneficial effect, delaying the age of onset.
First among them is an almost forgotten allele of the Apolipoprotein E (APOE) gene. APOE was identified in 1994 as the most important risk factor in sporadic, late-onset AD.
The gene, which is involved in cholesterol metabolism, has three major alleles: APOE*E2, which occurs in 7% of the general population; APOE*E3 (79%); and APOE*E4 (14%). The APOE*E4 allele carries the highest risk of late-onset AD of any mutation involved in sporadic AD.
The study by Arcos-Burgos and his colleagues directs the spotlight to another allele, APOE*E2, revealing it to be the most important determinant of the age of onset of AD in the Paisa cohort.
For Paisa family members with the pathogenic PSEN1 mutation, possession of a protective APO*E2 allele can delay the age at which they begin to exhibit AD symptoms by as much as 17 years. But everyone inherits two alleles of the APOE gene — one from each parent — and Arcos-Burgos and his colleagues found that in affected Paisa family members, the protective effect of APOE*E2 is negated if it is co-inherited with an APOE*E4 allele.
The protective effect of the APOE*E2 allele in affected Paisa family members also depends to a lesser degree on which particular alleles of the other eight genes identified by Arcos-Burgos’ team they might inherit — they variously delay or advance the age of onset of AD. The modifying effect of these secondary genes is known as epistasis.
The pathogenic influence of the APOE*E4 allele is not limited to the Paisa family — Arcos-Burgos says it is also the major risk factor for sporadic, late-onset forms of AD in the general population. Similarly, in the general population, just 2% of people who inherit the APOE*E2 allele will develop Alzheimer’s disease in their lifetimes.
“These epistatic interactions shape the life history of individuals at genetic risk of Alzheimer’s disease — they determine the age of onset, which symptoms they will exhibit and the type of AD they develop,” Arcos-Burgos said.
Among the nine genes in the network, the team found that a variant of the GRP20 (G protein-coupled receptor) gene also has a significant effect on the age of onset of AD in the Paisa cohort, probably through an interaction with APOE*E2. The epistatic interactions are potentially modifiable with targeted drugs or other therapies that would delay AD by enhancing their beneficial effects, or repress pathogenic effects.
In Australia, 24,700 people aged 65 or younger have early-onset dementia. An estimated 342,800 people in Australia are living with some form of dementia, the majority of whom have some form of AD, and this number is expected to rise to 900,000 by mid-century.
Blood test for chronic fatigue syndrome developed
The test addresses the need for a quick and reliable diagnostic for a complex,...
Droplet microfluidics for single-cell analysis
Discover how droplet microfluidics is revolutionising single-cell analysis and selection in...
PCR alternative offers diagnostic testing in a handheld device
Researchers have developed a diagnostic platform that uses similar techniques to PCR, but within...



