Putting the brain back into brain research
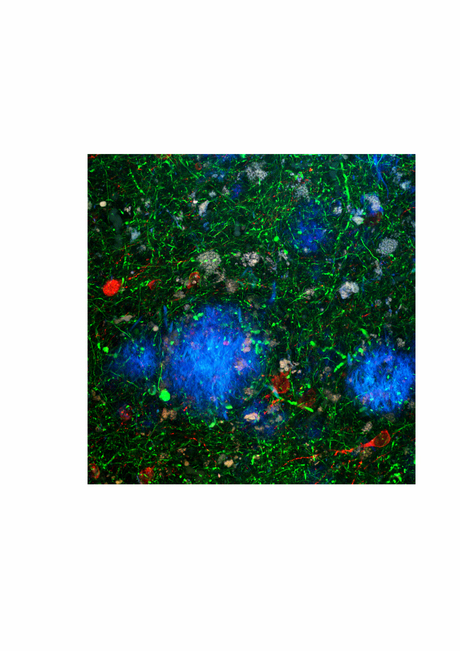
Facilities that store human brain tissue, so-called ‘brain banks’, are finding it harder than ever to get a share of the medical research dollar in Australia. Yet they remain an incredibly important, relevant and valuable resource for many studies in neuroscience, and Tasmanian neuroscientist James Vickers is keen to explain why.
Professor James Vickers is co-director of the Wicking Dementia Research and Education Centre, a University of Tasmania facility housed in a spiffy new building in downtown Hobart.
Established in 2008 to provide a unique combination of translational research and support specifically for issues surrounding dementia, the activities at the centre are incredibly diverse encompassing health services, lab-based neuroscience studies, research with carers and nursing staff, a Dementia Massive Open Online Course and, most recently, a long-term cohort study called the Tasmanian Healthy Brain Project.
Dementia is not a single disease, but rather a broad syndrome comprising a spectrum of degenerative disorders affecting the brain. It manifests as a progressive functional decline in thinking, memory, problem-solving skills and social behaviour - eventually affecting all aspects of everyday life.
Dementia has a profound impact on individuals, families and communities - even a glance at the statistics on dementia are quite sobering. In Australia, over 320,000 people are living with dementia and around 1.2 million are caring for someone with dementia. These numbers will increase each year as our population ages. Most startlingly, in less than 50 years, spending on dementia is set to outstrip that of any other health condition.
A bank you can trust
Vickers’ own 20 years of research has focused on Alzheimer’s disease, the most common form of dementia. His group conducts laboratory-based research using cell culture and transgenic animal models of the disease as well as human brain tissue - and it is the latter aspect he wants to highlight at the ANS meeting in January.
Vickers is part of a session spruiking the lessons to be learned from human brain research and the value of modern brain banking. “There is still so much we can learn only by looking at the human brain at various stages of a disease,” he said.
Much of the early research on Alzheimer’s disease in the 1980s and 1990s centred on establishing the pathological changes that progressively accumulate during the course of the disease by studying post-mortem human brain tissue. Indeed, the degenerative changes of Alzheimer’s disease may be present many years before any clinical symptoms appear.
“By that time, a whole bunch of nerve cells and synapses have degenerated irreversibly,” Vickers explained, “and from there it only gets worse, which can happen quite quickly over 2-3 years or it can take a decade or more.”
Fully understanding that pathological course was therefore crucial for establishing diagnostic criteria and early clinical management strategies for Alzheimer’s disease.
The new wave
Most of the laboratory studies on Alzheimer’s disease, which is probably the single biggest area neuroscience research these days, are heavily based around disease models, both transgenic animals and cell culture. Vickers estimates that a whole generation of scientists who have developed their career in Alzheimer’s disease research may not have spent any time actually looking at the brain tissue of affected humans or considering the substantial literature of what actually happens in the human brain during the disease.
This is not to say that the disease model studies are not incredibly important and informative - they are often the only way to really get at the biochemical and genetic mechanisms at play in diseases like Alzheimer’s. But what Vickers wants to do at ANS is remind everybody how valuable human brain tissue studies are within the overall neuroscience research picture.
“Human brain studies tell us for example that Alzheimer’s disease progresses very slowly in terms of how nerve cells degenerate and that it affects only a particular subset of cells and synapses - we call that selective vulnerability - but probably many model-based studies do not reference that framework,” said Vickers.
“For instance, there is a whole lot of literature around cell culture models of Alzheimer’s disease that involve acute poisoning of nerve cells, and that kills a lot of cells quickly, but trying to relate that back to how nerve cells die during the human disease can be quite difficult. Also, as the transgenic models become more and more sophisticated over time, how closely do they actually model the whole of Alzheimer’s disease, or are we instead studying parts of the disease process or pathology that may indeed be specific for a particular model?”
Resistance may not be futile
The other thing that Vickers will mention in his talk is the significant clinical inter-individual variability observed in Alzheimer’s disease and how difficult it is to relate this particular aspect to the experimental models.
“For instance, patients with the same amount of pathology may be affected quite differently - one person may be severely demented while another person with almost identical pathological changes is much less affected.”
What is unknown, however, is the basis for that variability. Is it genetic, do we build up different levels of cognitive resilience or brain reserve during our lifespan that have an effect and do risk factors affect the disease response?
“The thing with the models, and transgenic lines in particular, is that by the very nature of them there is not a lot of variability. While this is a good thing with many laboratory-based investigations, again you always have to keep the clinical disease story in the back of your mind.”
Getting the bigger picture
In a final key example, Vickers will present some of his own group’s results.
In a study published this year in the Journal of Neurochemistry, his team looked at synapse vulnerability in Alzheimer’s disease, both in human brain tissue at different pathological stages and in a commonly used transgenic model. It turned out that the transgenic mice developed the characteristic Alzheimer’s plaques in their brains but did not exhibit much neuronal degeneration, which is reminiscent of preclinical disease in humans before the dementia symptoms become really obvious.
Their analysis also revealed similar levels of synapse loss in brains from people with early-stage disease and those of the transgenic mice.
“So, rather than just saying something about established or end-stage disease, the whole story became so much more important for potential early interventions as a result of bringing in the human tissue comparison,” said Vickers. “Indeed, most of the transgenic models used in this field are not full models of Alzheimer’s disease but probably represent specific stages of the disease.”
This could actually become incredibly useful. Not only does it make any mechanistic studies using that model more relevant and powerful, but in the case of early-stage models, it could be very important for drug development.
“We know that a lot of Alzheimer’s drug trials have failed and that is probably because they have been focused on humans who just have too much pathology built up. So, now the whole field is moving towards trying to identify sufferers at the early stages of the disease as a better point of intervention.”
********************************************************
The Tasmanian Healthy Brain Project
The Tasmanian Healthy Brain Project (THBP) is a world-first, prospective longitudinal study of up to 1000 people aged 50-79 years to determine if tertiary education later in life reduces age-related cognitive decline and significantly decreases the risk or onset of dementia. Led by Vickers, the project commenced in 2011 and is planned to run for 10-20 years.
According to Vickers, there are increasing suggestions that complex mental stimulation could protect against ageing-related cognitive decline and that building up cognitive resilience might lower the risk of dementia.
“Basically, the older people in this study will undertake university study as a form of complex stimulation and then we will follow how that plays out in terms of cognitive measures … and eventually brain pathology in consenting subjects. We will also look at risk factors in these patients and genetic variations that have been implicated in loss of cognition and dementia - there is quite a bit of work going on currently in that area.”
********************************************************
Specially designed peptides can treat complex diseases
Two separate research teams have found ways to create short chains of amino acids, termed...
Exposure to aircraft noise linked to poor heart function
People who live close to airports could be at greater risk of poor heart function, increasing the...
Predicting the impact of protein mutations with simple maths
Researchers have discovered that the impact of mutations on protein stability is more predictable...



