Reprogrammed pancreatic cells relieve diabetes in mice
A new study led by the University of Geneva (UNIGE) has demonstrated how some human pancreatic cells can be reprogrammed to produce insulin, potentially compensating in diabetics for the loss or dysfunction of cells that naturally produce this hormone. Their work has been published in the journal Nature.
The human pancreas harbours several types of endocrine cells (α, β, δ, ε and ϒ) that produce different hormones responsible for regulating blood sugar levels. These cells are bundled into small clusters, called pancreatic islets or islets of Langerhans. Diabetes occurs when, in the absence of functional β cells, blood sugar levels are no longer controlled.
At the UNIGE Faculty of Medicine, Professor Pedro Herrera and his team had already demonstrated, in mice, that the pancreas has the ability to regenerate new insulin cells through a spontaneous mechanism of identity change of other pancreatic cells. But what about in human beings? And is it possible to artificially promote this conversion?
To explore whether human cells have this ability to adapt, the scientists used islets of Langerhans from both diabetic and non-diabetic donors. They first sorted the different cell types to study two of them in particular: α cells (glucagon producers) and ϒ cells (pancreatic polypeptide cells).
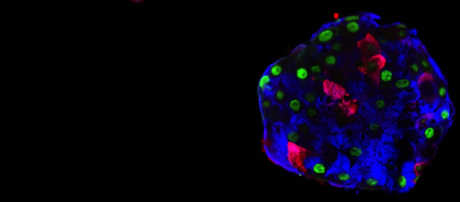
“We divided our cells into two groups: one where we introduced only a fluorescent cell tracer, and the other where, in addition, we added genes that produce insulin transcription factors specific to β cells,” Prof Herrera explained. The researchers then reconstructed ‘pseudo-islets’, with only one cell type at a time to accurately study their behaviour.
“First observation: the simple fact of aggregating cells, even into monotypic pseudo-islets, stimulates the expression of certain genes linked to insulin production, as if the ‘non-β’ cells naturally detected the absence of their ‘sisters’,” said Kenichiro Furuyama, a researcher at UNIGE and first author on the study. “However, in order for the cells to start producing insulin, we had to artificially stimulate the expression of one or two key β cell genes.”
One week after the experiment began, 30% of the α cells were producing and secreting insulin in response to glucose. ϒ cells, under the same treatment, were even more effective and numerous in converting and secreting insulin in response to glucose.
In a second step, the researchers transplanted these monotypic pseudo-islets of modified human α cells into diabetic mice. Prof Herrera recalled, “Human cells proved to be very effective — the mice recovered! And as expected, when these human cell transplants were removed the mice became diabetic again.
“We obtained the same results with cells from both diabetic and non-diabetic donors, showing that this plasticity is not damaged by the disease. In addition, this works in the long term: six months after transplantation, the modified pseudo-islets continued to secrete human insulin in response to high glucose.”
A detailed analysis of these human glucagon cells that have become insulin producers shows that they retain a cell identity close to that of α cells. With autoimmune diabetes (type 1 diabetes) characterised by the destruction of β cells by the immune system of patients, the researchers wondered whether these modified α cells would also be targeted by autoimmunity, since they remain different from β-cells.
To test their resistance, the researchers co-cultured these modified cells with T cells from patients with type 1 diabetes. They found that modified α cells triggered a weaker immune response, and therefore might be less likely to be destroyed than native β cells.
The researchers believe their research may one day be used as a substitute for pancreas transplantation, which is currently performed in cases of extremely severe diabetes and involves transplanting either the entire pancreas or, preferably, only pancreatic islets. This technique is very effective, but, like any transplant, it goes hand in hand with immunosuppressive treatment. The transplanted cells also disappear after a few years.
“The idea of using the intrinsic regenerative capacities of the human body makes sense here,” Prof Herrera said. However, many hurdles remain before a treatment resulting from the team’s discovery can be proposed.
“We must indeed find a way — pharmacological or by gene therapy — to stimulate this change of identity in the cells concerned within the patient’s own pancreas, but without causing adverse effects on other cell types.”
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletters and bimonthly magazine.
Mini lung organoids could help test new treatments
Scientists have developed a simple method for automated the manufacturing of lung organoids...
Clogged 'drains' in the brain an early sign of Alzheimer’s
'Drains' in the brain, responsible for clearing toxic waste in the organ, tend to get...
World's oldest known RNA extracted from woolly mammoth
The RNA sequences are understood to be the oldest ever recovered, coming from mammoth tissue...





