The magic of molybdenum
Solving the structure of proteins is all in a day’s work for Dr Megan Maher, who is using X-ray crystallography to further our understanding of how cells acquire and use trace metals.
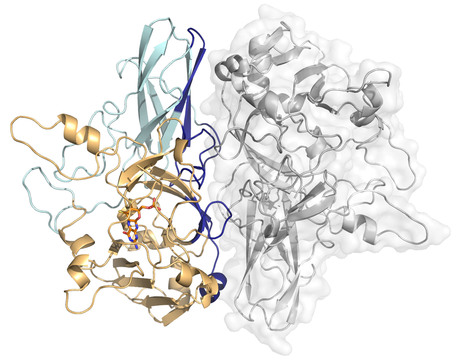
Patience, partnership and serendipity played their part for Dr Megan Maher and her team in solving the crystal structure of sulfite dehydrogenase (called SorT) and its electron acceptor.
Maher heads a lab in the La Trobe Institute for Molecular Science, at La Trobe University in Victoria. Her lab’s interest is in the role transition metals play in biology, with a particular focus on the technique of X-ray crystallography.
“Most people know about the requirement most organisms have for iron but fewer people are aware of the seemingly obscure elements like molybdenum and manganese, which are also required by all forms of life,” said Maher.
“My lab is interested in all aspects of that requirement. Everything from how a cell or organism acquires those trace metal nutrients through to how they are used in the various cell functions.”
The work Maher will present at the Lorne Protein meeting is part of a long-term collaboration with microbiologist/biochemist Dr Ulrike Kappler, at the University of Queensland, that is investigating how the trace metal molybdenum is used in bacterial respiration.
Kappler identifies enzymes from bacteria that use molybdenum and characterises their activities and biochemical properties, while Maher uses X-ray crystallography to determine their structures.
Essential for life
Trace metals, such as iron, copper, magnesium and molybdenum, occur in extremely small quantities in plant and animal cells. All cells require them and they occur in soil and water in the environment.
“Molybdenum is essential for all forms of life,” Maher said. “There are a few specialised bacteria that will use tungsten instead but most forms of life require molybdenum.”
According to Maher, about 30% of proteins and enzymes require a transition metal component for activity. Transition metals are required for the structural integrity or stabilisation of a protein, or they are needed for an enzyme to be active.
Molybdenum is incorporated into enzymes and facilitates reactivities that an enzyme would otherwise be unable to access using amino acids alone. This is related to molybdenum’s ability to redox cycle.
“Molybdenum can access three oxidation states in the biological range,” explained Maher. “It can accept or donate two electrons at a time to a reaction - no other transition metal or elements can do that.”
A reactive species
In humans there are four enzyme systems so far characterised that rely on molybdenum, including xanthine oxidase, which is important in uric acid synthesis and sulfite oxidase, which converts sulfite to sulfate. Maher’s team is working on the latter enzyme in bacteria.
“The sulfite oxidase enzymes are required for sulfite detoxification in all organisms,” explained Maher. “For example, in humans, sulfite results from the breakdown of sulfur-containing amino acids in normal cellular processes of metabolism, such as cysteine and methionine.
Sulfite is very reactive and needs to be quickly converted to sulfate to prevent it from damaging cells - for example, it reacts readily with protein and DNA. This is the role of sulfite oxidase and it would not be able to do it without molybdenum.
Using sulfite for energy production
Maher’s latest work has involved looking at the equivalent enzyme, SorT in the bacterium, Sinorhizobium meliloti, which is found on the root nodules of legumes. This bacterium can live on sulfur-containing compounds, using the sulfite-sulfate conversion for energy generation.
“The bacterial system is simpler to work with in terms of generating the protein and purifying it to do the types of studies we want to do, rather than working on the human enzyme,” she said.
Isolating the enzyme from its bacterial source does not generate very much pure protein, so Kappler cloned the enzyme into E. coli and developed a method to generate quantities of recombinant protein for Maher’s X-ray crystallography.
“We need milligram quantities of pure protein to grow crystals,” Maher explained.
The electron transfer dance
But it’s not just SorT Maher’s team has been trying to crystallise, they have also been looking for its partner or electron acceptor in the cycling reaction.
When molybdenum accepts the two electrons during sulfite oxidation, it then interacts with another protein as an electron acceptor, enabling the enzymatic reaction to take place again. Much like the mitochondrial electron transfer chain, the fundamental process driving ATP synthesis in cells, the electrons flow from one protein to another through a chain.
“Given it is such as fundamental process, we still don’t entirely understand what governs it,” said Maher. “In the electron transport chain of the mitochondria, the protein-protein complexes are located close together in the mitochondrial membrane, but in the case of SorT and its electron acceptor, they float around in the cell periplasm.
“They need to find each other and interact very specifically but very transiently - the interaction cannot be so specific that they cannot separate again because electron transfer has to be very fast.”
Growing crystals
Not many electron transfer structures have been solved because of the transience of the interaction between the two molecules. But Maher’s team got lucky and successfully crystallised SorT with its electron acceptor.
Now that they have solved the structure it will help us understand how this class of enzyme functions.
“We were very lucky to get the structure of the complex,” Maher recalled. “We basically mixed the two proteins together, put them into our crystallisation experiments and crossed our fingers - and we got one crystal.
“Often, because of the requirement for that fine balance between a specific but not very strong interaction, it’s very difficult to crystallise the two proteins in complex because they need to come together for some time in order to pack into a crystal.”
Once they captured the transient interaction, they took their prize crystal to the Australian synchrotron where they have access to the high-intensity radiation needed for X-ray crystallography.
“We wouldn’t be able to do this work without the synchrotron,” said Maher, recalling times when her research was restricted to experiments twice a year because it required visiting a synchrotron overseas.
X-ray crystallography literally reveals the packing of the molecules within a crystal. A diffraction pattern is produced that can be used to generate an electron density map, which is basically a 3D contour map of where the electrons are positioned in the crystal which, in turn, is where the atoms are.
“Once we generate this electron density map, it’s like a big puzzle, we then need to interpret where each atom is in this beautiful net,” said Maher. “We can actually see exactly where the atoms are and once we interpret that map we can see the structure of SorT and its electron acceptor together in the crystal.”
Solving the structure
Solving the structure of the complex not only increases our understanding of the molybdenum class of enzymes, it will help the researchers better understand the electron transfer process - how two proteins can come together specifically but also transiently. This is relevant to all electron transfer processes that occur in cells because they all have the same requirement.
“The more we understand about their structures and their functions, the more we understand about the important processes they carry out,” said Maher. “These results have reinforced a lot of the theory that was out there already about electron transfer complexes. It’s very nice that it made sense. A lot of theoretical work has been done and it all married up very nicely.”
**********************************************************
Lorne conference line-up
Here’s the line-up for the Lorne conferences for 2014, to be held at Mantra Lorne on the Victorian south coast.
19th Lorne Proteomics Symposium
February 6-9
http://www.australasianproteomics.org/lorne-proteomics-symposium-2014/
39th Lorne Conference on Protein Structure and Function
February 9-13
http://www.lorneproteins.org/
26th Lorne Cancer Conference
February 13-15
http://www.lornecancer.org/
35th Lorne Genome Conference
February 16-19
http://www.lornegenome.org/
Lorne Infection and Immunity
February19-21
www.lorneinfectionimmunity.org
**********************************************************
AI-designed DNA switches flip genes on and off
The work creates the opportunity to turn the expression of a gene up or down in just one tissue...
Drug delays tumour growth in models of children's liver cancer
A new drug has been shown to delay the growth of tumours and improve survival in hepatoblastoma,...
Ancient DNA rewrites the stories of those preserved at Pompeii
Researchers have used ancient DNA to challenge long-held assumptions about the inhabitants of...




